
Compare the structures of \[{{H}_{2}}O\] and \[{{H}_{2}}{{O}_{2}}\] .
Answer
595.2k+ views
Hint: In both the structures there are only single bonds present. The bond angle has changed due to the presence of lone pairs. Due to their bonding, they can form a complicated structure.
Complete step by step solution:
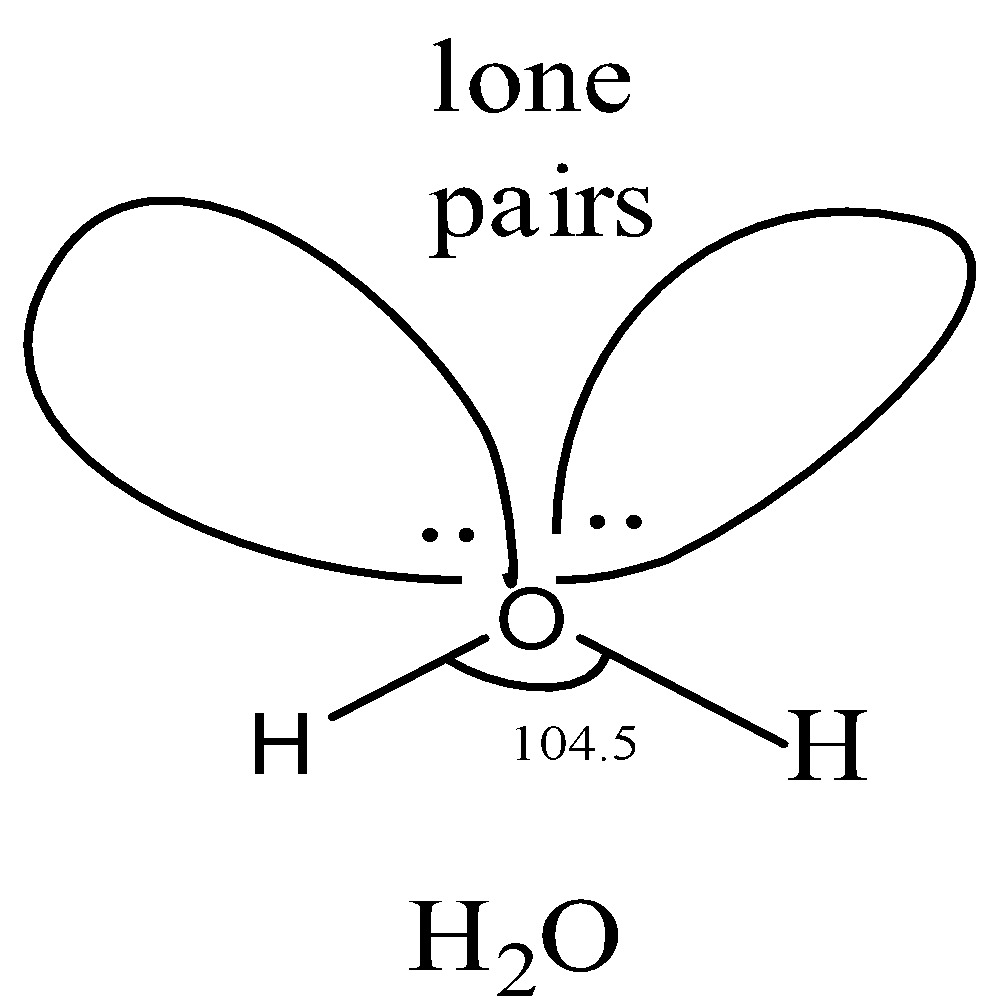
Let us study the structure of \[{{H}_{2}}O\].
In \[{{H}_{2}}O\]molecule, the oxygen atom is and hence has four \[s{{p}^{3}}-hybridized\] orbitals. Two of these\[s{{p}^{3}}-orbitals\] are half-filled and hence overlap with hydrogen to form two while the other two contain a lone pair of electrons each. Since the oxygen atom is \[s{{p}^{3}}-hybridized\], therefore, the geometry of the molecule should be tetrahedral and the bond angle should be \[{{109.5}^{\circ }}\]. But experimentally, the oxygen atom is surrounded by two shared pairs, and two lone pairs of the electron. But according to VSEPR theory, lone pair-lone pair repulsion is greater than bond pair-bond pair repulsion. As a result, the angle decreases to\[{{104.5}^{\circ }}\].
The bond length is 95.7pm.
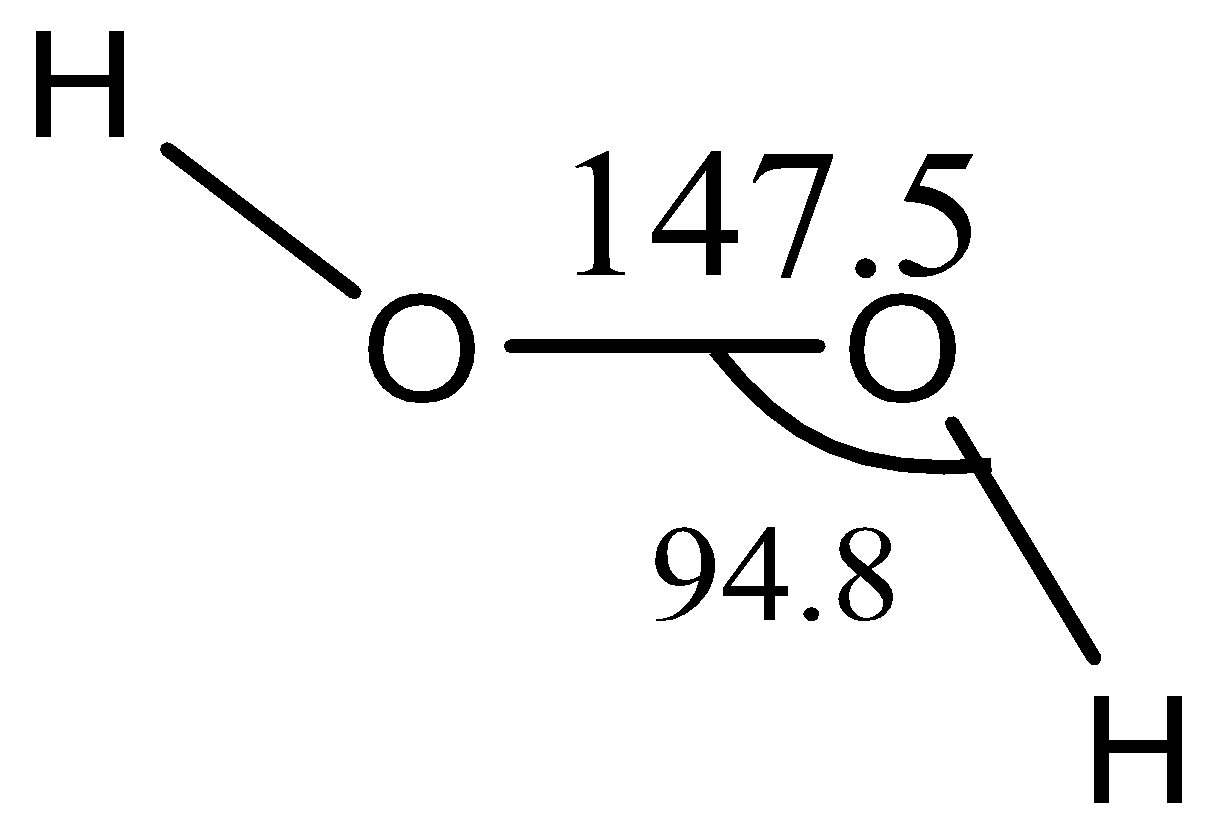
Now, the structure of \[{{H}_{2}}{{O}_{2}}\]
Hydrogen peroxide is a nonpolar molecule. The two oxygen atoms are linked to each other by a single covalent bond and each oxygen is further linked to a hydrogen atom by a single bond. The two bonds are, however, in different planes due to repulsion between different bonding and antibonding orbitals. The dihedral angle between the two planes being \[{{111.5}^{\circ }}\]and in the gas phase reduces to \[{{90.2}^{\circ }}\]. The bond length is 147.5pm and 95pm.
Note: Don’t get confused that if the hybridization is then the shape would be tetrahedral, lone pairs repulsion is a factor that should be considered. Water has a planar structure whereas hydrogen peroxide has a non-planar structure.
Complete step by step solution:
Let us study the structure of \[{{H}_{2}}O\].
In \[{{H}_{2}}O\]molecule, the oxygen atom is and hence has four \[s{{p}^{3}}-hybridized\] orbitals. Two of these\[s{{p}^{3}}-orbitals\] are half-filled and hence overlap with hydrogen to form two while the other two contain a lone pair of electrons each. Since the oxygen atom is \[s{{p}^{3}}-hybridized\], therefore, the geometry of the molecule should be tetrahedral and the bond angle should be \[{{109.5}^{\circ }}\]. But experimentally, the oxygen atom is surrounded by two shared pairs, and two lone pairs of the electron. But according to VSEPR theory, lone pair-lone pair repulsion is greater than bond pair-bond pair repulsion. As a result, the angle decreases to\[{{104.5}^{\circ }}\].
The bond length is 95.7pm.

Now, the structure of \[{{H}_{2}}{{O}_{2}}\]
Hydrogen peroxide is a nonpolar molecule. The two oxygen atoms are linked to each other by a single covalent bond and each oxygen is further linked to a hydrogen atom by a single bond. The two bonds are, however, in different planes due to repulsion between different bonding and antibonding orbitals. The dihedral angle between the two planes being \[{{111.5}^{\circ }}\]and in the gas phase reduces to \[{{90.2}^{\circ }}\]. The bond length is 147.5pm and 95pm.

Note: Don’t get confused that if the hybridization is then the shape would be tetrahedral, lone pairs repulsion is a factor that should be considered. Water has a planar structure whereas hydrogen peroxide has a non-planar structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




