
How can I compare the structural formula and sawhorse projection of 2-bromo-3-chlorobutane?
Answer
535.5k+ views
Hint: Stereoisomers of compounds have the ability to have a spatial arrangement in space and are called conformers. They have a free rotation of carbon that arranges in different orientations in space giving us various projections.
Complete answer:
The compound 2-bromo-3-chlorobutane contains a 4-carbon chain along with bromide and chloride group at adjacent, 2 and 3 carbons respectively.
The structural formula of 2-bromo-3-chlorobutane can be made as:
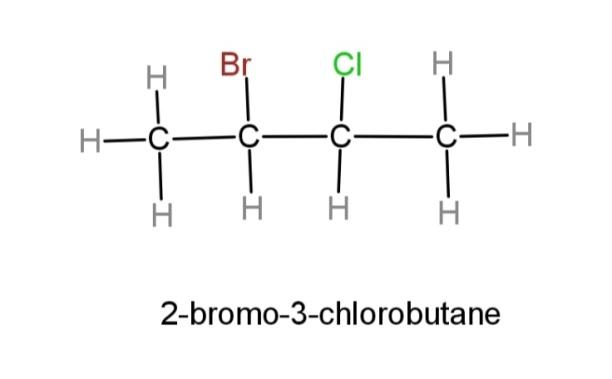
The structural formula is a straight chain of carbon along with bromide and chloride groups. This structure does not tell us about any stereochemical aspect of the compound, which is its spatial arrangement in space.
Sawhorse projection is a method to show the spatial arrangement of atoms in a compound as follows:
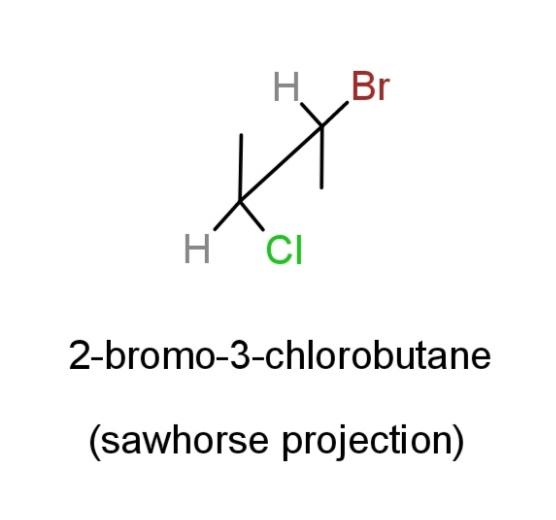
This sawhorse projection structure tells us that the methyl groups are attached at both the ends, showing the bond at the vertical line. While the bromide group is attached at carbon-2, and chloride group at carbon-3. This also shows that carbon-2 and carbon-3 have chirality and therefore, four stereoisomers can be derived from 2-bromo-3-chlorobutane.
Hence, the structural formula of 2-bromo-3-chlorobutane does not tell any of its stereochemistry, while the sawhorse projection structure tells us the stereochemistry of this compound.
Note:
Sawhorse projection can be of two types, staggered projection and eclipsed projection. Here eclipsed projection is shown. The projection becomes staggered when both the end group lines are vertically upwards.
Complete answer:
The compound 2-bromo-3-chlorobutane contains a 4-carbon chain along with bromide and chloride group at adjacent, 2 and 3 carbons respectively.
The structural formula of 2-bromo-3-chlorobutane can be made as:

The structural formula is a straight chain of carbon along with bromide and chloride groups. This structure does not tell us about any stereochemical aspect of the compound, which is its spatial arrangement in space.
Sawhorse projection is a method to show the spatial arrangement of atoms in a compound as follows:

This sawhorse projection structure tells us that the methyl groups are attached at both the ends, showing the bond at the vertical line. While the bromide group is attached at carbon-2, and chloride group at carbon-3. This also shows that carbon-2 and carbon-3 have chirality and therefore, four stereoisomers can be derived from 2-bromo-3-chlorobutane.
Hence, the structural formula of 2-bromo-3-chlorobutane does not tell any of its stereochemistry, while the sawhorse projection structure tells us the stereochemistry of this compound.
Note:
Sawhorse projection can be of two types, staggered projection and eclipsed projection. Here eclipsed projection is shown. The projection becomes staggered when both the end group lines are vertically upwards.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




