
Compare the best sequence of the reactions for transformations given. Semicolons indicate separate reaction steps to be used in the order shown.
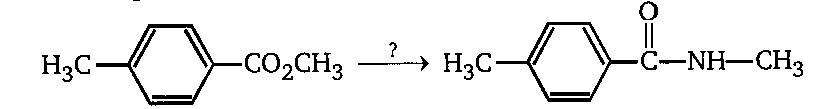
A. \[{H_3}{O^ + };SOC{l_2};C{H_3}N{H_2}\]
B. \[H{O^ - }/{H_2}O;PB{r_3};Mg;C{O_2};{H_3}{O^ - };SOC{l_2};C{H_3}N{H_2}\]
C. $LiAl{H_4}/{H_2}O;HBr;Mg;C{O_2};{H_3}{O^ + };SOC{l_2};C{H_3}N{H_2}$
D. None of these would yield the desired product.
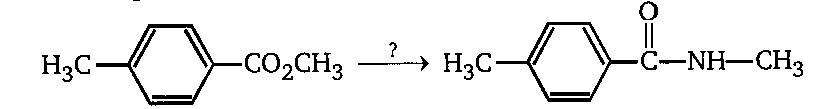
Answer
588k+ views
Hint: The best way to follow this type of reaction is to start thinking of products made up by reversing the reaction. Any organic acid on hydrolysis gives carboxylic acid. If acyl chloride is reacted with amines then the resultant product will be amide.
Complete step by step answer:
In sequential reactions the way to solve the steps or find out the missing products is a very tricky thing. To start this type of reaction we can assume the reactants as the final product and proceed reversibly or we can proceed by the help of options.
We will use options to solve this problem. We will check every option and when we get our desired product we will end the checking.
So we are starting with option A.
As we have given that reactant is Methoxy acid, we will introduce \[{H_3}{O^ + }\] to it.
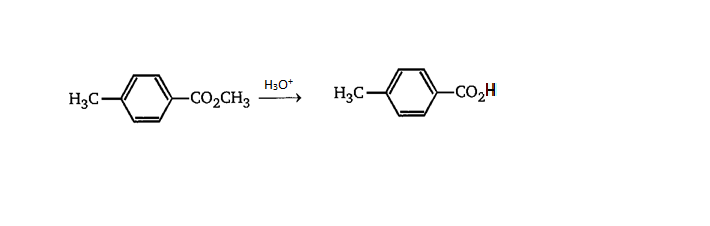
In the above reaction we can see that when hydrolysis is done of a methoxy group it converts itself into carboxylic acid.
Further we will react with the obtained carboxylic acid with thionyl chloride.
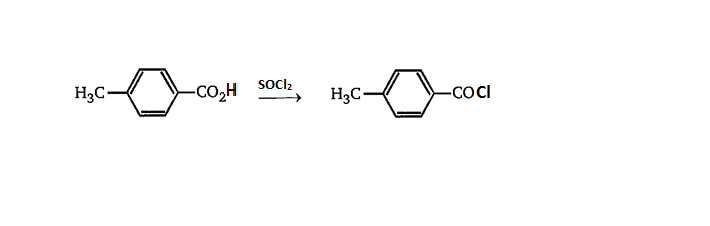
When thionyl chloride reacts with carboxylic acid it gives acyl chloride.
Now the Acyl chloride will react with amine,
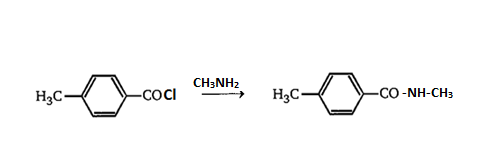
The reaction of acyl chloride with amine produces our desired product.
So, the correct answer is “Option A”.
Note: When carboxylic acid reacts with thionyl chloride, acid chloride is produced. During the reaction the sulphur present in thionyl chloride converts the hydroxyl group into an intermediate named chlorosulfite which is a good leaving group and the chloride ion acts as a nucleophile and finally acid chloride is produced.
Complete step by step answer:
In sequential reactions the way to solve the steps or find out the missing products is a very tricky thing. To start this type of reaction we can assume the reactants as the final product and proceed reversibly or we can proceed by the help of options.
We will use options to solve this problem. We will check every option and when we get our desired product we will end the checking.
So we are starting with option A.
As we have given that reactant is Methoxy acid, we will introduce \[{H_3}{O^ + }\] to it.

In the above reaction we can see that when hydrolysis is done of a methoxy group it converts itself into carboxylic acid.
Further we will react with the obtained carboxylic acid with thionyl chloride.

When thionyl chloride reacts with carboxylic acid it gives acyl chloride.
Now the Acyl chloride will react with amine,

The reaction of acyl chloride with amine produces our desired product.
So, the correct answer is “Option A”.
Note: When carboxylic acid reacts with thionyl chloride, acid chloride is produced. During the reaction the sulphur present in thionyl chloride converts the hydroxyl group into an intermediate named chlorosulfite which is a good leaving group and the chloride ion acts as a nucleophile and finally acid chloride is produced.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




