
What is the common between three states of matter?
A.They are made up of small tiny particles.
B.They have a particular mass and can occupy space.
C.These three states have volume in it.
D.The atoms of these three states have a force of attractions between them.
Answer
582.6k+ views
Hint: Matter can be classified into different states on the basis of intermolecular forces and the arrangement of particles such as solid, liquid and gas. Matter is something that has volume and can occupy space. It is composed of small particles such as atoms or molecules.
Complete step by step answer:
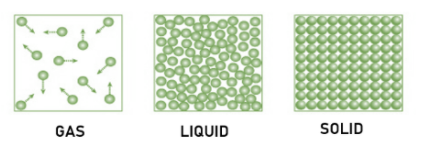
-Solid: The particles are tightly packed together in the solid as the forces between the particles are intense such that the particles cannot move freely but can only vibrate. This makes the solid stable with definite shape and a certain volume. Solids differ from liquids and gases by the characteristic feature of rigidity. The molecules of solids are tightly packed due to strong intermolecular forces and they only oscillate about their mean positions. Solids have the least compressibility and thermal expansion such as iron.
-Liquid: It is an incompressible fluid that takes the shape of its container but retains a nearly constant volume independent of the pressure. The molecules in a liquid are closely packed because of weak intermolecular forces. These are weaker than solids but stronger than that of gases. There is much space in between the molecules of liquids and this makes their flowing ability easy. We can also know that the density of liquid lies between the density of solids and gases. Also, we can learn that compressibility and thermal expansion of liquids are slightly higher than that of solids such as water.
-Gas: The molecules have enough kinetic energy due to which impact of the intermolecular forces is small and the normal distance between the adjacent molecules is much more than the molecular size. Gases do not have definite shape or volume, but it occupies the entire container in which it is confined. They experience negligible intermolecular forces and do not have any fixed shape or volume. They also have high compressibility and thermal expansion. For example, oxygen gas.
Hence, the correct options are (A), (B), (C) and (D).
Note:
There are many other states other than these three, such as Bose – Einstein condensate and neutron degenerate matter occur only in extreme conditions such as ultra-cold or ultra-dense matter. Also, quark – gluon plasmas are known to be possible but remain theoretical for the time being.
Complete step by step answer:
-Solid: The particles are tightly packed together in the solid as the forces between the particles are intense such that the particles cannot move freely but can only vibrate. This makes the solid stable with definite shape and a certain volume. Solids differ from liquids and gases by the characteristic feature of rigidity. The molecules of solids are tightly packed due to strong intermolecular forces and they only oscillate about their mean positions. Solids have the least compressibility and thermal expansion such as iron.
-Liquid: It is an incompressible fluid that takes the shape of its container but retains a nearly constant volume independent of the pressure. The molecules in a liquid are closely packed because of weak intermolecular forces. These are weaker than solids but stronger than that of gases. There is much space in between the molecules of liquids and this makes their flowing ability easy. We can also know that the density of liquid lies between the density of solids and gases. Also, we can learn that compressibility and thermal expansion of liquids are slightly higher than that of solids such as water.
-Gas: The molecules have enough kinetic energy due to which impact of the intermolecular forces is small and the normal distance between the adjacent molecules is much more than the molecular size. Gases do not have definite shape or volume, but it occupies the entire container in which it is confined. They experience negligible intermolecular forces and do not have any fixed shape or volume. They also have high compressibility and thermal expansion. For example, oxygen gas.

Hence, the correct options are (A), (B), (C) and (D).
Note:
There are many other states other than these three, such as Bose – Einstein condensate and neutron degenerate matter occur only in extreme conditions such as ultra-cold or ultra-dense matter. Also, quark – gluon plasmas are known to be possible but remain theoretical for the time being.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




