
$C{O_2}$ is gas while $Si{O_2}$ is solid because:
A: $C{O_2}$ is a linear molecule, while $Si{O_2}$ is agular.
B: van der waals’ forces are very strong in $Si{O_2}$.
C: $C{O_2}$ is covalent, while $Si{O_2}$ is ionic.
D: $Si$ cannot form stable bonds with $O$, hence $Si$ has to form a $3D$ lattice.
Answer
583.2k+ views
Hint: Carbon and silicon both belong to the same group in the periodic table. This means these atoms have similar properties. These atoms have a tendency to show catenation. Carbon shows catenation to the maximum extent.
Complete step by step answer:
Carbon and silicon both belong to the same group in the periodic table. This means these atoms have similar properties. These atoms have a tendency to show catenation. Catenation is the property of an element with which it can form a long chain by linking with other atoms of the same element. In $C{O_2}$ there is a double bond between carbon and oxygen atoms. Carbon has a tendency to make bonds with oxygen but silicon cannot make bonds with oxygen due to which it forms a giant molecular network with oxygen. Structure of this molecule is as follows:
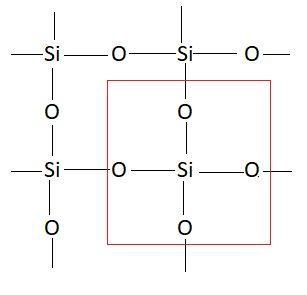
Due to this the bonding in this molecule is very strong and hence it exists as solid. This means due to the inability of silicon atoms to make bonds with oxygen $Si{O_2}$ make a giant network. In this network bonding is strong and hence this exists as solid. So, the correct answer is option D that is $Si$ cannot form stable bonds with $O$, hence $Si$ has to form a $3D$ lattice.
So, the correct answer is Option D.
Note:
Catenation is the property of an element with which it makes bonds with other atoms of the same element. Carbon shows maximum catenation. Size of carbon is small due to which the hold of the nucleus of the shared pair of electrons is more and it is able to make long chains.
Complete step by step answer:
Carbon and silicon both belong to the same group in the periodic table. This means these atoms have similar properties. These atoms have a tendency to show catenation. Catenation is the property of an element with which it can form a long chain by linking with other atoms of the same element. In $C{O_2}$ there is a double bond between carbon and oxygen atoms. Carbon has a tendency to make bonds with oxygen but silicon cannot make bonds with oxygen due to which it forms a giant molecular network with oxygen. Structure of this molecule is as follows:

Due to this the bonding in this molecule is very strong and hence it exists as solid. This means due to the inability of silicon atoms to make bonds with oxygen $Si{O_2}$ make a giant network. In this network bonding is strong and hence this exists as solid. So, the correct answer is option D that is $Si$ cannot form stable bonds with $O$, hence $Si$ has to form a $3D$ lattice.
So, the correct answer is Option D.
Note:
Catenation is the property of an element with which it makes bonds with other atoms of the same element. Carbon shows maximum catenation. Size of carbon is small due to which the hold of the nucleus of the shared pair of electrons is more and it is able to make long chains.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




