
How many Cl−Cl bond(s) is/are present in $ C{l_2}{O_7} $
Answer
509.1k+ views
Hint :A chemical bond is a long-term attraction between atoms, ions, or molecules that allows chemical compounds to form. Ionic bonds are formed by the electrostatic force of attraction between oppositely charged ions, whereas covalent bonds are formed by the sharing of electrons. Chemical bonds come in a variety of strengths; there are "strong bonds" or "primary bonds" like covalent, ionic, and metallic connections, as well as "weak bonds" or "secondary bonds" like dipole–dipole interactions, the London dispersion force, and hydrogen bonding.
Complete Step By Step Answer:
The chemical compound $ C{l_2}{O_7} $ stands for dichlorine heptoxide. The anhydride of perchloric acid is chlorine oxide. Perchloric acid is carefully distilled in the presence of the dehydrating agent phosphorus pentoxide to create it:
$ 2{\text{ }}HCl{O_4}\; + {\text{ }}{P_4}{O_{10}}\; \to {\text{ }}C{l_2}{O_7}\; + {\text{ }}{H_2}{P_4}{O_{11}} $
The chlorine(VII) oxide in the mixture may be distilled off. Illumination on chlorine and ozone mixes can also produce it. When anhydrous, it slowly hydrolyzes back to perchloric acid, which is similarly dangerous. $ C{l_2}{O_7} $ is an endergonic molecule, which means it's intrinsically unstable and breaks down into its constituent atoms when energy is released. $ C{l_2}{O_7} $ is bent at $ {118.6^o} $ with the $ Cl - O - Cl $ angle, producing the molecule $ {C_2} $ symmetry. The terminal $ Cl = O $ distances are $ 1.709 $ , while the $ Cl = O $ distances are $ 1.405 $ . Although the bonding in this molecule is largely covalent, chlorine lives at its maximum formal oxidation state of +7 in this chemical.
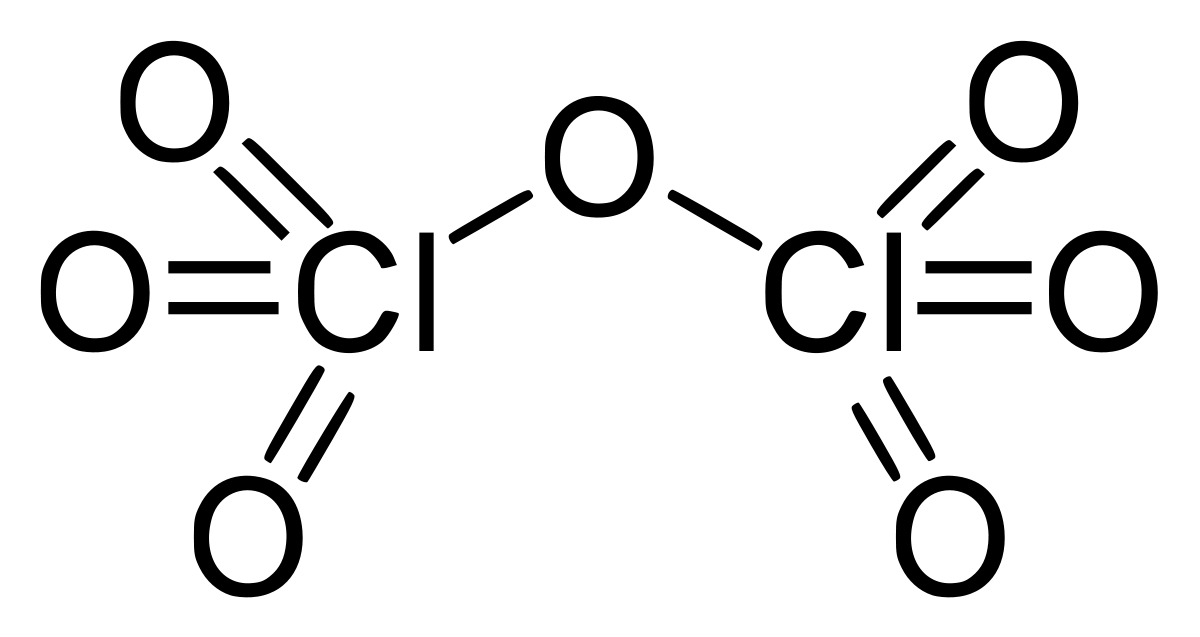
here no two chlorines are getting attached.
Hence the answer is zero.
Note :
$ C{l_2}{O_7} $ , although being the most stable chlorine oxide, is a powerful oxidant and explosive that may be ignited by flame, mechanical force, or contact with iodine. It is, however, less oxidising than the other chlorine oxides, and when cold, it does not damage sulphur, phosphorus, or paper. It has the same effects on the human body as elemental chlorine and must be handled with the same care.
Complete Step By Step Answer:
The chemical compound $ C{l_2}{O_7} $ stands for dichlorine heptoxide. The anhydride of perchloric acid is chlorine oxide. Perchloric acid is carefully distilled in the presence of the dehydrating agent phosphorus pentoxide to create it:
$ 2{\text{ }}HCl{O_4}\; + {\text{ }}{P_4}{O_{10}}\; \to {\text{ }}C{l_2}{O_7}\; + {\text{ }}{H_2}{P_4}{O_{11}} $
The chlorine(VII) oxide in the mixture may be distilled off. Illumination on chlorine and ozone mixes can also produce it. When anhydrous, it slowly hydrolyzes back to perchloric acid, which is similarly dangerous. $ C{l_2}{O_7} $ is an endergonic molecule, which means it's intrinsically unstable and breaks down into its constituent atoms when energy is released. $ C{l_2}{O_7} $ is bent at $ {118.6^o} $ with the $ Cl - O - Cl $ angle, producing the molecule $ {C_2} $ symmetry. The terminal $ Cl = O $ distances are $ 1.709 $ , while the $ Cl = O $ distances are $ 1.405 $ . Although the bonding in this molecule is largely covalent, chlorine lives at its maximum formal oxidation state of +7 in this chemical.

here no two chlorines are getting attached.
Hence the answer is zero.
Note :
$ C{l_2}{O_7} $ , although being the most stable chlorine oxide, is a powerful oxidant and explosive that may be ignited by flame, mechanical force, or contact with iodine. It is, however, less oxidising than the other chlorine oxides, and when cold, it does not damage sulphur, phosphorus, or paper. It has the same effects on the human body as elemental chlorine and must be handled with the same care.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




