
Choose the correct order of decreasing basic strength of the following compounds in aqueous solution - (a) \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\] (b) \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\] (c) $N{{H}_{3}}$ (d) \[{{(C{{H}_{3}})}_{2}}NH\]
(A) (a) > (b) > (c) > (d)
(B) (d) > (b) > (c) > (a)
(C) (b) > (a) > (c) > (d)
(D) (d) > (c) > (b) > (a)
(E) (b) > (d) > (c) > (a)
Answer
548.4k+ views
Hint: Consider inductive effect and resonance effect of alkyl groups and benzyl groups. This can be used to predict the order of basic strength of the compounds.
Complete step by step solution:
- For understanding and comparing the basicity of ammonia and derivatives (amines), we have to consider the Inductive effect of alkyl and benzyl groups, and the resonance effect of benzyl groups. We also need to remember that the basicity of an amine is determined by the availability of the lone pair on the N atom.
- Let us first consider the inductive effect. According to the inductive effect, molecules containing groups that donate electrons will increase basicity and groups that decrease electron density will decrease its basicity.
- Alkyl groups are electron donating +I effect groups. Secondary amines will be more basic than primary amines as there are 2 alkyl groups directly attached in secondary amines which contribute more to the electron density.
-Benzyl groups are electron-withdrawing –I affect groups. Moreover, in aniline, there will be a resonance effect (– R) that decreases the net electron density on the N atom.
- We can understand this by looking at the resonance structures of aniline.
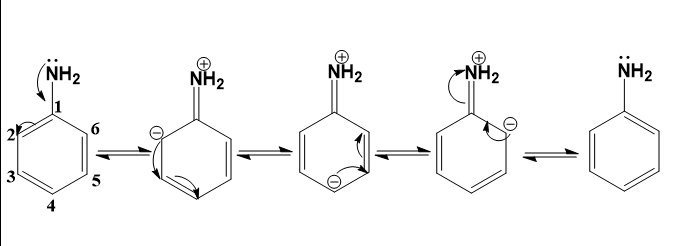
We can see that the lone pair of electrons that are responsible for basicity get delocalised into the ring.
- We can also infer that the basicity of ammonia will be more than that of aniline (as it contains a –I and –R effect group) and less than that of alkyl amines (as they contain +I effect groups).
- The other factor is the solvation effect where the solubility of dimethylamine is more as they have polar bonds to form hydrogen bonding with water.
- Ethylamine is less soluble in water compared to dimethylamine as the extent of hydrogen bond formation is less because of the presence of large ethyl group compared to small methyl groups.
- Solubility of ammonia is high in water but less compared to the other two mentioned above as ammonia is not substituted by the alkyl chains.
- Aniline is insoluble in water as it has a large hydrophobic group.
- Hence, from all the above information, the decreasing order will be –
\[{{(C{{H}_{3}})}_{2}}NH\] > \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\] > $N{{H}_{3}}$ > \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
- Therefore, we can conclude that the answer is option (B) (d) > (b) > (c) > (a).
Note:
- You should be careful that you remember to consider all the effects applicable to that question. You might get the wrong answer if you only consider one of the effects. Also, if the aqueous medium is mentioned, you have to consider the solvation effect.
Complete step by step solution:
- For understanding and comparing the basicity of ammonia and derivatives (amines), we have to consider the Inductive effect of alkyl and benzyl groups, and the resonance effect of benzyl groups. We also need to remember that the basicity of an amine is determined by the availability of the lone pair on the N atom.
- Let us first consider the inductive effect. According to the inductive effect, molecules containing groups that donate electrons will increase basicity and groups that decrease electron density will decrease its basicity.
- Alkyl groups are electron donating +I effect groups. Secondary amines will be more basic than primary amines as there are 2 alkyl groups directly attached in secondary amines which contribute more to the electron density.
-Benzyl groups are electron-withdrawing –I affect groups. Moreover, in aniline, there will be a resonance effect (– R) that decreases the net electron density on the N atom.
- We can understand this by looking at the resonance structures of aniline.

We can see that the lone pair of electrons that are responsible for basicity get delocalised into the ring.
- We can also infer that the basicity of ammonia will be more than that of aniline (as it contains a –I and –R effect group) and less than that of alkyl amines (as they contain +I effect groups).
- The other factor is the solvation effect where the solubility of dimethylamine is more as they have polar bonds to form hydrogen bonding with water.
- Ethylamine is less soluble in water compared to dimethylamine as the extent of hydrogen bond formation is less because of the presence of large ethyl group compared to small methyl groups.
- Solubility of ammonia is high in water but less compared to the other two mentioned above as ammonia is not substituted by the alkyl chains.
- Aniline is insoluble in water as it has a large hydrophobic group.
- Hence, from all the above information, the decreasing order will be –
\[{{(C{{H}_{3}})}_{2}}NH\] > \[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\] > $N{{H}_{3}}$ > \[{{C}_{6}}{{H}_{5}}N{{H}_{2}}\]
- Therefore, we can conclude that the answer is option (B) (d) > (b) > (c) > (a).
Note:
- You should be careful that you remember to consider all the effects applicable to that question. You might get the wrong answer if you only consider one of the effects. Also, if the aqueous medium is mentioned, you have to consider the solvation effect.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




