
Chloroform is used:
A. As a general anesthetic
B. As a solvent for fats and oils
C. As a preservatives for biological specimens
D. All are correct
Answer
559.2k+ views
Hint: We need to remember that the chloroform (also known as trichloromethane or methane trichloride) chemical formula is $CHC{l_3}$ . Chloroform is a dense liquid, colourless and strong smelling compound. Chloroform vapours on ignition with a green edged flame. Human and rabbit eyes and also the skin of the rabbits was irritated by neay chloroform.
Complete step by step answer:
We must know that the chloroform is a clear, volatile liquid at room temperature. Chloroform is prepared industrially by the partial reduction of carbon tetrachloride $(CC{l_4})$ with iron filings and water and this reaction also gives $HCl$ . The chemical equation is as follows,
$CC{l_4} + 2H\xrightarrow{{Fe + {H_2}O}}CHC{l_3} + HCl$
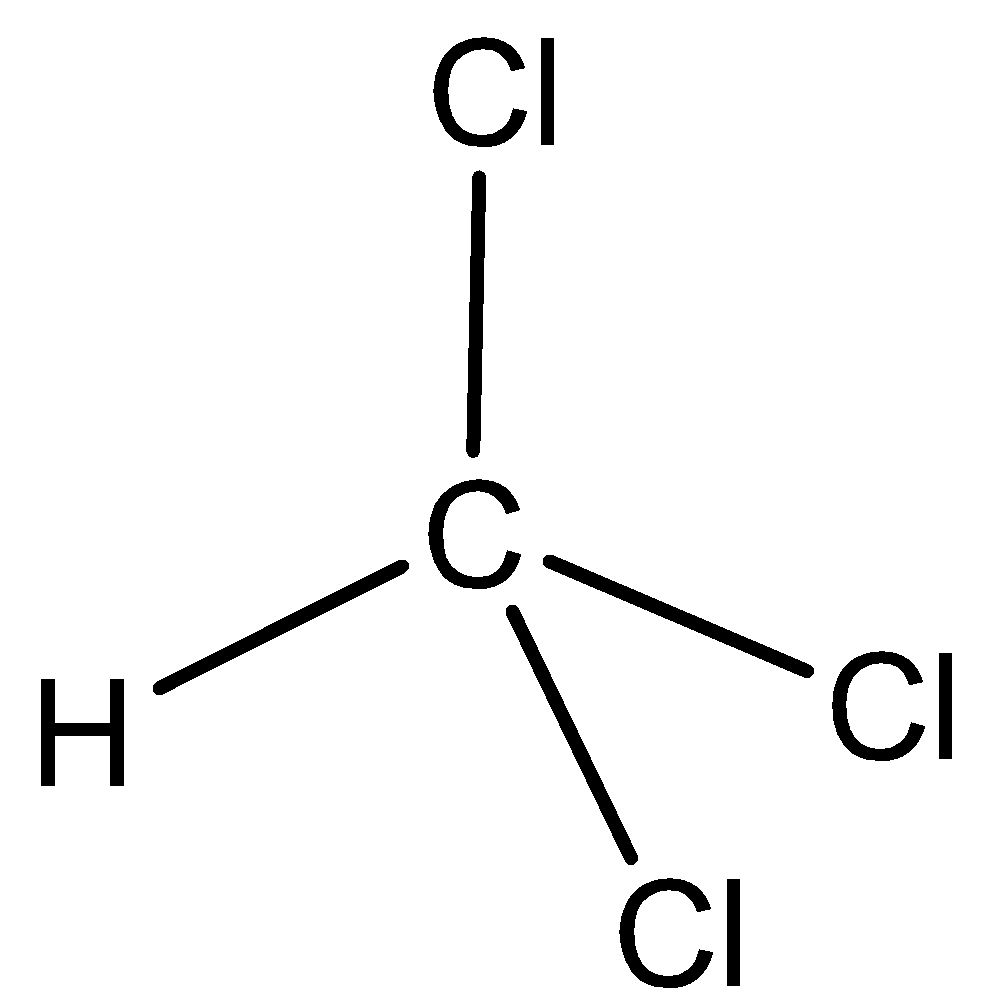
The chemical structure of chloroform is,
Let us see the choices one by one to seek out the right answer.
Chloroform as an anesthetic
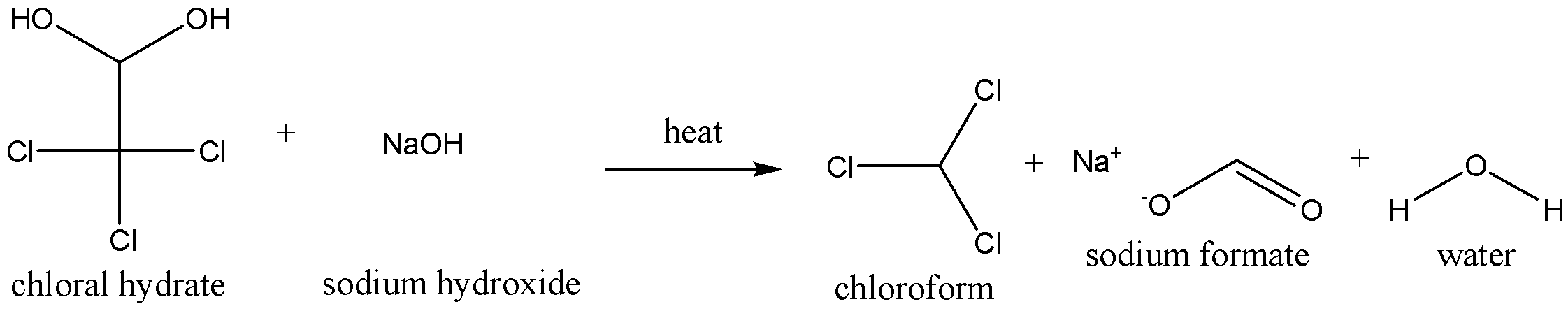
We need to know that the chloroform is used as an anesthetic (a drug, causing temporary loss of bodily sensations) to some extent. As an anesthetic pure chloroform is prepared by distilling chloral hydrate with conc. aqueous solution of caustic soda (sodium hydroxide).
Chloroform as a solvent
We have to remember that the chloroform is used as a solvent for fat, oils, iodine, waxes and resins.
Chloroform as a preservative
Chloroform is used as a preservative with ethyl alcohol$({C_2}{H_5}OH)$, for biological anatomical specimens.
From the above information chloroform is used as a general anesthetic, as a solvent for fats and oils and also used as a preservative for biological specimens.
So, the correct answer is Option D.
Note: We must remember that due to its toxicity to liver and kidney chloroform not used as an anesthetic in now-a-days. Chloroform hepatotoxicity and nephrotoxicity is assumed to flow from largely to phosgene. The discharge of chloroform ( $\left( {12\mu g/{m^2}} \right)$ per day) from an organic-rich spruce forest soil, under aerobic conditions within the laboratory, suggested biogenic formation.
Complete step by step answer:
We must know that the chloroform is a clear, volatile liquid at room temperature. Chloroform is prepared industrially by the partial reduction of carbon tetrachloride $(CC{l_4})$ with iron filings and water and this reaction also gives $HCl$ . The chemical equation is as follows,
$CC{l_4} + 2H\xrightarrow{{Fe + {H_2}O}}CHC{l_3} + HCl$
The chemical structure of chloroform is,

Let us see the choices one by one to seek out the right answer.
Chloroform as an anesthetic
We need to know that the chloroform is used as an anesthetic (a drug, causing temporary loss of bodily sensations) to some extent. As an anesthetic pure chloroform is prepared by distilling chloral hydrate with conc. aqueous solution of caustic soda (sodium hydroxide).

Chloroform as a solvent
We have to remember that the chloroform is used as a solvent for fat, oils, iodine, waxes and resins.
Chloroform as a preservative
Chloroform is used as a preservative with ethyl alcohol$({C_2}{H_5}OH)$, for biological anatomical specimens.
From the above information chloroform is used as a general anesthetic, as a solvent for fats and oils and also used as a preservative for biological specimens.
So, the correct answer is Option D.
Note: We must remember that due to its toxicity to liver and kidney chloroform not used as an anesthetic in now-a-days. Chloroform hepatotoxicity and nephrotoxicity is assumed to flow from largely to phosgene. The discharge of chloroform ( $\left( {12\mu g/{m^2}} \right)$ per day) from an organic-rich spruce forest soil, under aerobic conditions within the laboratory, suggested biogenic formation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




