
What is the chemical formula of iodine crystals?
Answer
494.4k+ views
Hint: Iodine is the chemical element which is represented by a symbol $I$ and its atomic number is $53$ . It is the heaviest element of the halogen group. It is semi-lustrous, non-metallic solid at standard conditions which melts at ${114^ \circ }C$ to form a deep violet liquid and boils at ${184^ \circ }C$ to form a violet gas.
Complete answer:
Iodine is a dark grey or purplish colour element whose atomic number is $53$. It is the part of the halogen group in the periodic table. Iodine was first of all discovered by French chemist Dr.Bernard Courtois while he was trying to burn the seaweed and got violet coloured ashes along with sulfuric acid as a by-product in the experiment, named it “Chemical X”. After that Sir Humphry Davy went to Italy, on his way he found this “chemical X” has similar properties to that of chlorine and named it Iodine, which means violet in Greek.
The atomic number of Iodine is 53 and its valency is $ - 1$ . It exists in nature as a diatomic molecule, i.e. its molecule contains two atoms of iodine which are connected by covalent bonds.
Covalent bond is the sharing of electrons between two or more atoms so that the overall molecule becomes stable. Here the Iodine atom shares one electron with another Iodine atom making the overall molecule stable. The chemical formula of iodine is ${I_2}$ .
The structure of the iodine molecule, ${I_2}$ is:
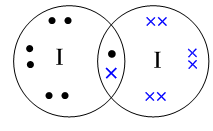
Note:
Iodine is an important element needed for life. It is known for the vital role it plays in the production of thyroid hormone in humans as well as in all vertebrates. Iodine deficiency can lead to serious health issues, like goiter, intellectual disability and cretinism.
Complete answer:
Iodine is a dark grey or purplish colour element whose atomic number is $53$. It is the part of the halogen group in the periodic table. Iodine was first of all discovered by French chemist Dr.Bernard Courtois while he was trying to burn the seaweed and got violet coloured ashes along with sulfuric acid as a by-product in the experiment, named it “Chemical X”. After that Sir Humphry Davy went to Italy, on his way he found this “chemical X” has similar properties to that of chlorine and named it Iodine, which means violet in Greek.
The atomic number of Iodine is 53 and its valency is $ - 1$ . It exists in nature as a diatomic molecule, i.e. its molecule contains two atoms of iodine which are connected by covalent bonds.
Covalent bond is the sharing of electrons between two or more atoms so that the overall molecule becomes stable. Here the Iodine atom shares one electron with another Iodine atom making the overall molecule stable. The chemical formula of iodine is ${I_2}$ .
The structure of the iodine molecule, ${I_2}$ is:

Note:
Iodine is an important element needed for life. It is known for the vital role it plays in the production of thyroid hormone in humans as well as in all vertebrates. Iodine deficiency can lead to serious health issues, like goiter, intellectual disability and cretinism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




