
What is the chemical form for barium bromate?
Answer
524.4k+ views
Hint: Barium is a chemical element with the symbol 'Ba' and the atomic number of Ba is 56. Barium is not found in nature as a free element because of its high reactivity. Similarly, bromine is another element that has the symbol 'Br' with an atomic number 35 which is also highly reactive hence which is not found in nature as a free element.
Complete step by step answer:
We have to know that the chemical form of barium bromate is \[Ba{\left( {Br{O_3}} \right)_2}\] with molecular mass, Barium bromate is a white crystalline powder or powder which is denser than water and it is faintly soluble in water. When it is reacted with organic compounds, it may explode and it is used as a corrosion inhibitor.
We can draw the structure of barium bromate as,
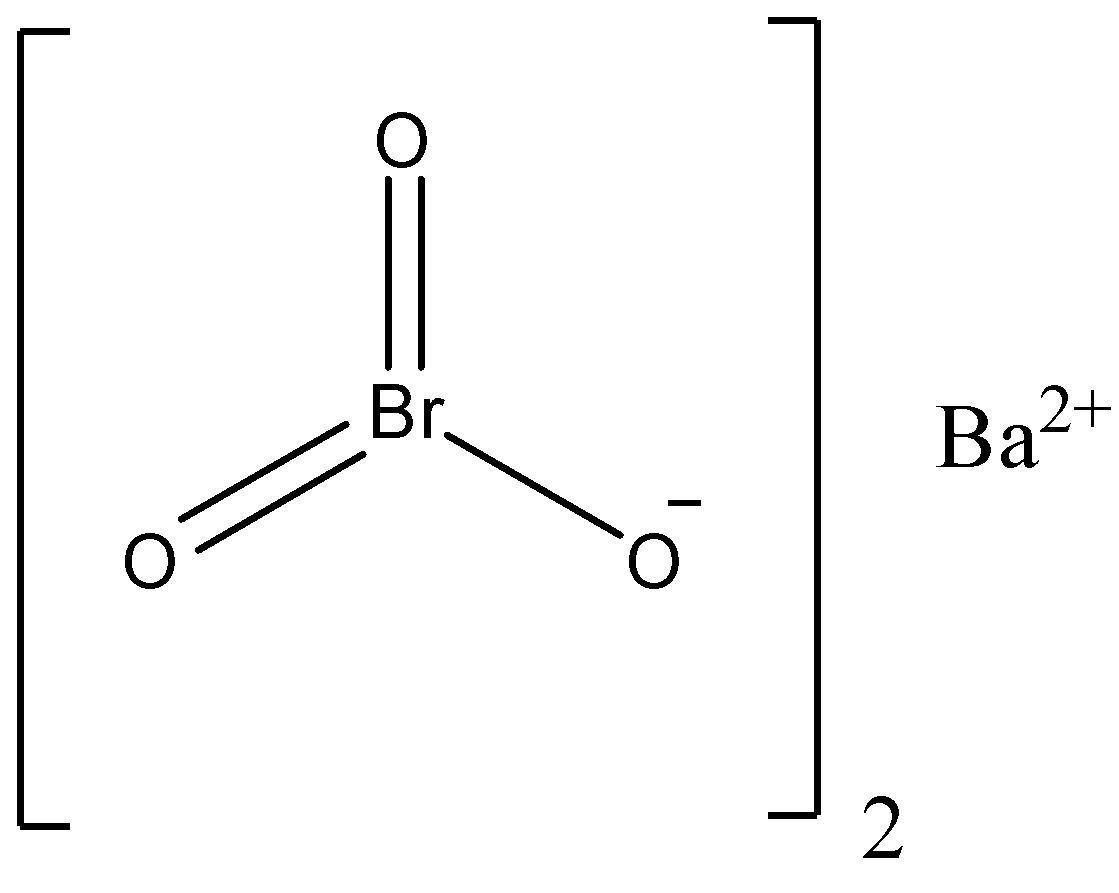
We must know that the barium bromate is mainly used as an oxidizing agent and analytical reagent and it is widely used to prepare other bromates like bromic acid, alkali metal bromates, etc. The barium bromate can be prepared by the reaction of hydroxide with sodium bromate and the reaction can be written as,
\[Ba{\left( {OH} \right)_2} + 2NaBr{O_3} \to Ba{\left( {Br{O_3}} \right)_2} + 2NaOH\]
Note: Barium bromate is a white crystalline solid and the chemical form for barium bromate can be written as \[Ba{\left( {Br{O_3}} \right)_2}\]. We have to remember that the barium bromate is slightly soluble in water and is mainly used as a corrosion inhibitor.
Complete step by step answer:
We have to know that the chemical form of barium bromate is \[Ba{\left( {Br{O_3}} \right)_2}\] with molecular mass, Barium bromate is a white crystalline powder or powder which is denser than water and it is faintly soluble in water. When it is reacted with organic compounds, it may explode and it is used as a corrosion inhibitor.
We can draw the structure of barium bromate as,

We must know that the barium bromate is mainly used as an oxidizing agent and analytical reagent and it is widely used to prepare other bromates like bromic acid, alkali metal bromates, etc. The barium bromate can be prepared by the reaction of hydroxide with sodium bromate and the reaction can be written as,
\[Ba{\left( {OH} \right)_2} + 2NaBr{O_3} \to Ba{\left( {Br{O_3}} \right)_2} + 2NaOH\]
Note: Barium bromate is a white crystalline solid and the chemical form for barium bromate can be written as \[Ba{\left( {Br{O_3}} \right)_2}\]. We have to remember that the barium bromate is slightly soluble in water and is mainly used as a corrosion inhibitor.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




