
$C{{H}_{4}}$ has:
a.) Linear Geometry
b.) Bent geometry
c.) Tetrahedral geometry
d.) Pyramidal geometry
Answer
569.1k+ views
Hint: Geometry of the molecule depends on two factors. One is hybridization and second is number of lone present in central atom. As in Carbon there will not be a lone pair for the given molecule, that’s why it's geometry will purely be decided on the basis of hybridization alone. In case of $sp$ hybridization, a molecule will have linear geometry and in case of $s{{p}^{3}}$ hybridization molecule will have tetrahedral geometry. Since Carbon doesn’t have any lone pair, therefore it can’t have bent or pyramidal geometry.
Complete Solution :
Electronic configuration for of Carbon:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$
- As carbon has to accommodate 4 hydrogen atoms, therefore it needs 4 half filled orbitals, so one 2s electron will be transferred into 2p orbital. Now new electronic configuration is:
$1{{s}^{2}}2{{s}^{1}}2{{p}^{3}}$
Now it has one s orbital and 3 p orbitals as half filled orbitals and no lone pair in valence shell, therefore these 4 orbitals will take part in hybridization, so there will be clearly $s{{p}^{3}}$ hybridization.
We know that molecule that has $s{{p}^{3}}$ hybridization without any lone pair, always has tetrahedral geometry.
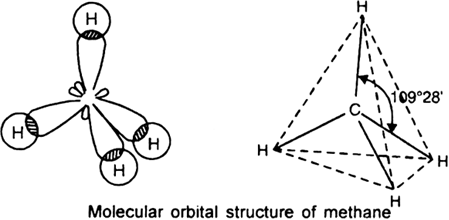
So $C{{H}_{4}}$ has tetrahedral geometry.
So, the correct answer is “Option C”.
Additional Information:
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. It has the bond angle of ${{109}^{0}}{{28}^{'}}$. It is a symmetrical shape.
Note: Methane is gas that is found in small quantities in Earth's atmosphere. Methane is a powerful greenhouse gas. Methane is flammable, and is used as a fuel worldwide. It can explode at concentrations between 5% (lower explosive limit) and 15% (upper explosive limit). It is relatively non-toxic gas. Its health effects are associated with being simple asphyxiate displacing oxygen in the lungs.
Complete Solution :
Electronic configuration for of Carbon:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$
- As carbon has to accommodate 4 hydrogen atoms, therefore it needs 4 half filled orbitals, so one 2s electron will be transferred into 2p orbital. Now new electronic configuration is:
$1{{s}^{2}}2{{s}^{1}}2{{p}^{3}}$
Now it has one s orbital and 3 p orbitals as half filled orbitals and no lone pair in valence shell, therefore these 4 orbitals will take part in hybridization, so there will be clearly $s{{p}^{3}}$ hybridization.
We know that molecule that has $s{{p}^{3}}$ hybridization without any lone pair, always has tetrahedral geometry.

So $C{{H}_{4}}$ has tetrahedral geometry.
So, the correct answer is “Option C”.
Additional Information:
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. It has the bond angle of ${{109}^{0}}{{28}^{'}}$. It is a symmetrical shape.
Note: Methane is gas that is found in small quantities in Earth's atmosphere. Methane is a powerful greenhouse gas. Methane is flammable, and is used as a fuel worldwide. It can explode at concentrations between 5% (lower explosive limit) and 15% (upper explosive limit). It is relatively non-toxic gas. Its health effects are associated with being simple asphyxiate displacing oxygen in the lungs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




