
Catalytic hydrogenation of which of the following will yield isopentane?
A.
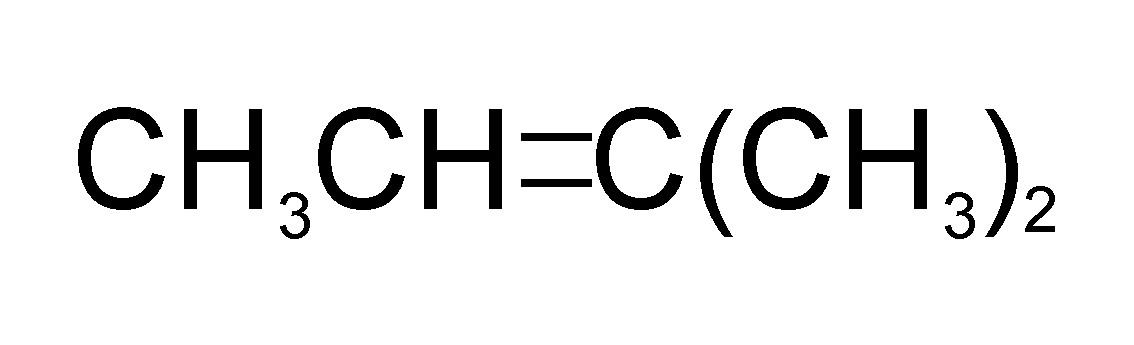
B.
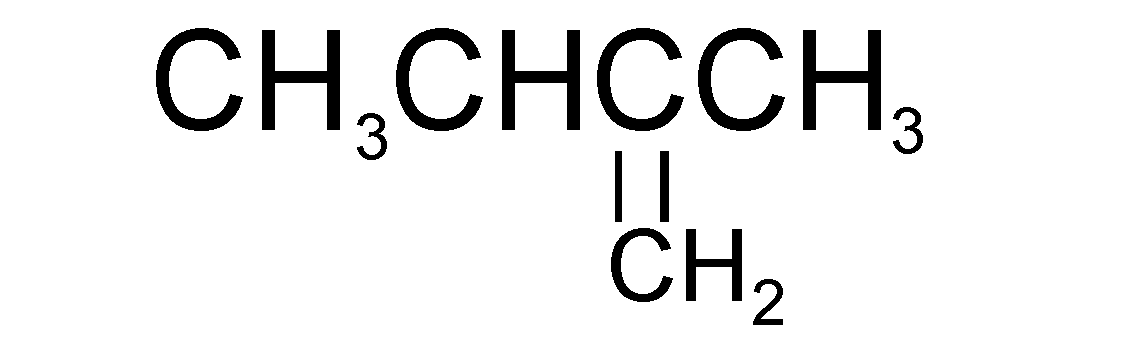
C.
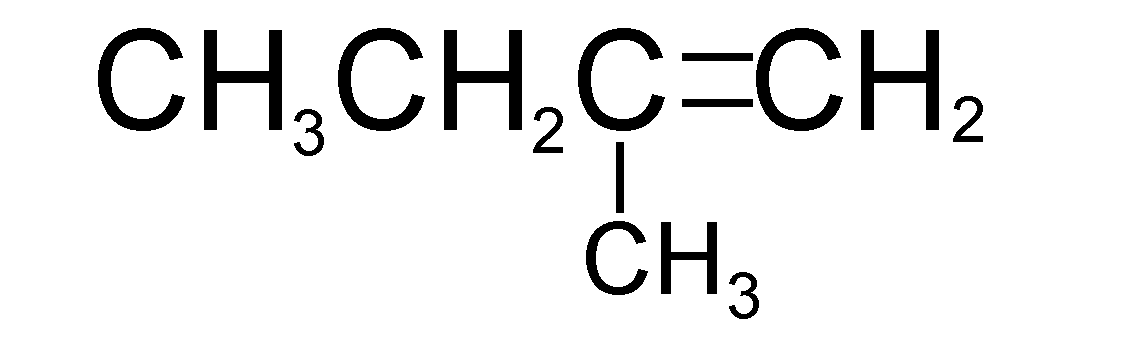
D.
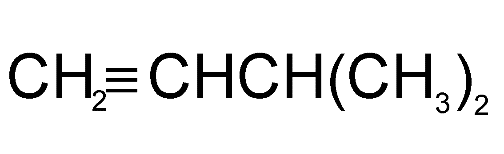
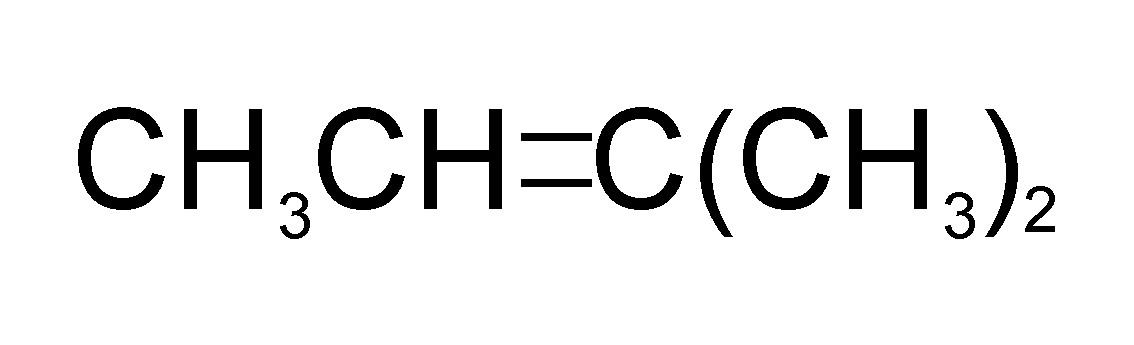
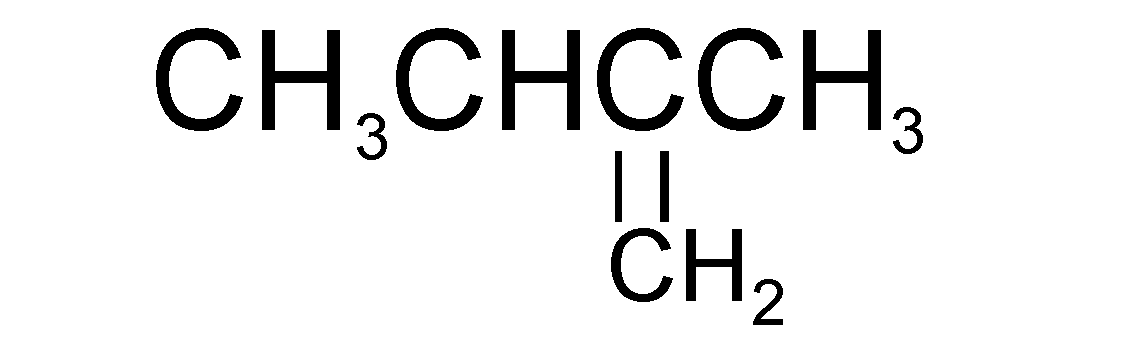
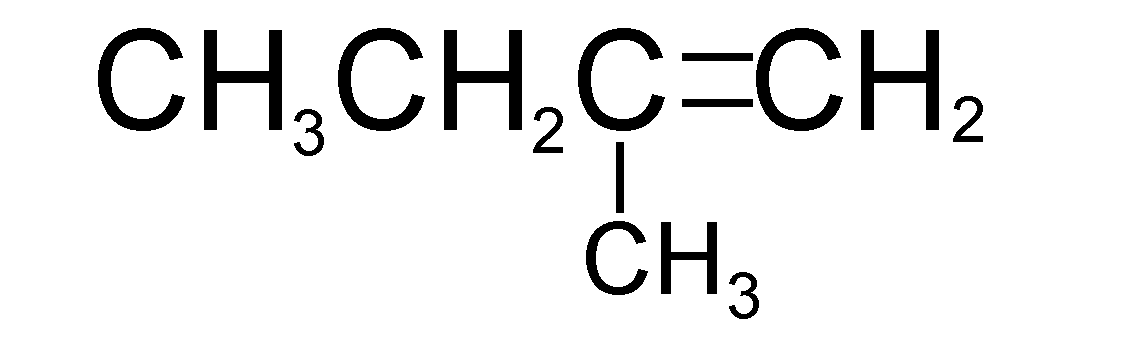

Answer
550.8k+ views
Hint: Let’s start by discussing the catalytic hydrogenation reaction.
Hydrogenation reaction – Hydrogenation is a chemical reaction, normally in the presence of a catalyst such as nickel, palladium, or platinum, between molecular hydrogen ( ${H_2}$ ) and another compound. This reaction can convert alkene to an alkane.
Complete answer:
Hydrogenation is a chemical reaction, normally in the presence of a catalyst such as nickel, palladium, or platinum, between molecular hydrogen (${H_2}$ ) and another compound or part. The method is widely used for the reduction or saturation of organic compounds. The addition of pairs of hydrogen atoms to a molecule, often an alkene, usually constitutes hydrogenation. For the reaction to be usable, catalysts are required; non-catalytic hydrogenation takes place only at very high temperatures.
There are three components of hydrogenation: the unsaturated substrate, the hydrogen (or source of hydrogen), and, invariably, the catalyst. Depending on the substrate and the catalyst's operation, the reduction reaction is carried out at various temperatures and pressures.
With occasional exceptions, in the absence of metal catalysts, H2 is unreactive against organic compounds. The unsaturated layer, with most sites protected by the substrate, is chemisorbed onto the catalyst. Hydrogen forms surface hydrides (M-H) in heterogeneous catalysts from which hydrogen can be transferred to the chemisorbed substratum. Highly active catalysts that work at lower temperatures and lower pressures of ${H_2}$ are formed by platinum, palladium, rhodium, and ruthenium.
The only molecule that can produce isopentane after undergoing catalytic hydrogenation is molecule A (i.e. 2-methylbut-2-ene)
The reaction is as follows
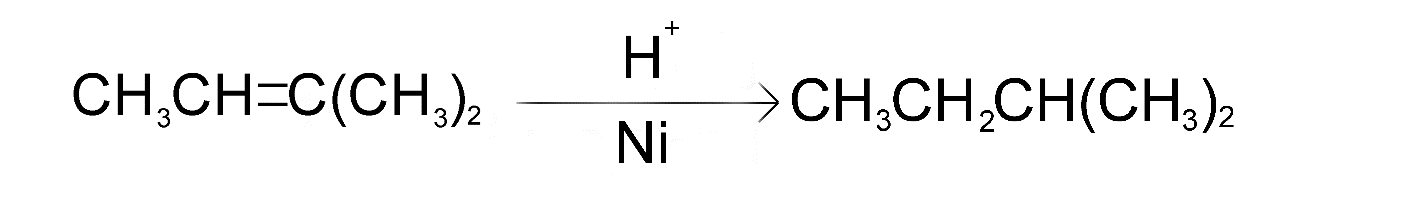
Hence, option A is the correct choice.
Note:
Non-precious metal catalysts have also been developed as economical alternatives, especially nickel-based ones (such as Raney nickel and Urushibara nickel), but they are often slower or require higher temperatures. The trade-off is operation (reaction speed) vs. catalyst cost and apparatus cost needed for high-pressure use.
Hydrogenation reaction – Hydrogenation is a chemical reaction, normally in the presence of a catalyst such as nickel, palladium, or platinum, between molecular hydrogen ( ${H_2}$ ) and another compound. This reaction can convert alkene to an alkane.
Complete answer:
Hydrogenation is a chemical reaction, normally in the presence of a catalyst such as nickel, palladium, or platinum, between molecular hydrogen (${H_2}$ ) and another compound or part. The method is widely used for the reduction or saturation of organic compounds. The addition of pairs of hydrogen atoms to a molecule, often an alkene, usually constitutes hydrogenation. For the reaction to be usable, catalysts are required; non-catalytic hydrogenation takes place only at very high temperatures.
There are three components of hydrogenation: the unsaturated substrate, the hydrogen (or source of hydrogen), and, invariably, the catalyst. Depending on the substrate and the catalyst's operation, the reduction reaction is carried out at various temperatures and pressures.
With occasional exceptions, in the absence of metal catalysts, H2 is unreactive against organic compounds. The unsaturated layer, with most sites protected by the substrate, is chemisorbed onto the catalyst. Hydrogen forms surface hydrides (M-H) in heterogeneous catalysts from which hydrogen can be transferred to the chemisorbed substratum. Highly active catalysts that work at lower temperatures and lower pressures of ${H_2}$ are formed by platinum, palladium, rhodium, and ruthenium.
The only molecule that can produce isopentane after undergoing catalytic hydrogenation is molecule A (i.e. 2-methylbut-2-ene)
The reaction is as follows
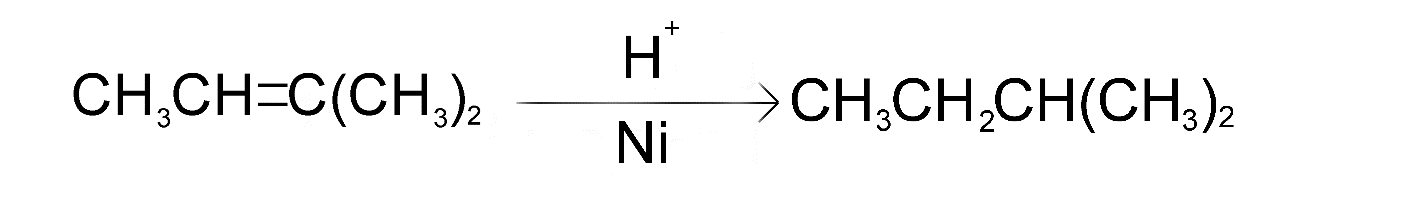
Hence, option A is the correct choice.
Note:
Non-precious metal catalysts have also been developed as economical alternatives, especially nickel-based ones (such as Raney nickel and Urushibara nickel), but they are often slower or require higher temperatures. The trade-off is operation (reaction speed) vs. catalyst cost and apparatus cost needed for high-pressure use.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




