
Catalyst used in dimerisation of acetylene to prepare chloroprene is
A . HgSO4 + H2SO4
B . Cu2Cl2
C . Cu2Cl2 + NH4Cl
D . Cu2Cl2 + NH4OH
Answer
345.9k+ views
Hint: In this question we have to use the reaction of dimerisation of acetylene to prepare chloroprene to find out the catalyst used in this reaction. Dimerization is the process by which two molecules with comparable chemical make-up combine to produce a single polymer called a dimer.
Complete Step by Step Answer:
When acetylene reacts with hydrogen chloride to create 4-chloro-1, 2-butadiene, an allene derivative, it dimerizes to produce vinyl acetylene. In the presence of cuprous chloride(Cu2Cl2) and ammonium chloride(NH4Cl), this derivative rearranges to produce 2-Chlorobuta-1, 3-diene.
There are two phases in the conventional synthesis of chloroprene. Acetylene is first dimerized to vinylacetylene in a reaction tower at 80 °C in an aqueous hydro-chloric acid solution of Cu2Cl2 and NH4Cl.
Water evaporation regulates the significant evolution of heat. The conversion of C2H2 is around 18%. The major by-product is divinylacetylene, and the selectivity to vinylacetylene can reach 90%. In the second stage HCl is added to vinylacetylene, forming chloropropene.
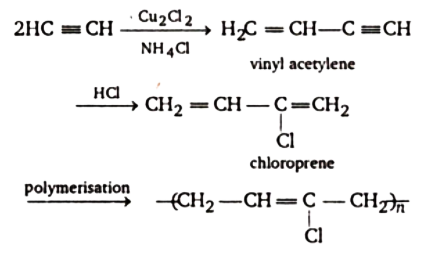
The correct answer is option (C) Cu2Cl2 + NH4Cl.
Additional Information: A catalyst is a substance that, without being consumed by the process, speeds up a chemical reaction or lowers the temperature or pressure required to initiate one. The addition of a catalyst to a reaction is known as catalysis. By lowering the activation energy, the energy barrier that must be cleared for a chemical reaction to take place, catalysts increase the efficiency of a reaction. There will be two steps in the conversion of acetylene to chloroprene. A triple bond and two carbon atoms are both present in the organic molecule acetylene. Four carbon atoms make up the chemical chloroprene, which also contains a chlorine atom in the second position. There are double bonds in the first and third positions.
Note: A triple bond and two carbon atoms are both present in the organic molecule acetylene. Four carbon atoms make up the chemical chloroprene, which also contains a chlorine atom in the second position. There will be two processes required to transform acetylene into chloroprene.
Complete Step by Step Answer:
When acetylene reacts with hydrogen chloride to create 4-chloro-1, 2-butadiene, an allene derivative, it dimerizes to produce vinyl acetylene. In the presence of cuprous chloride(Cu2Cl2) and ammonium chloride(NH4Cl), this derivative rearranges to produce 2-Chlorobuta-1, 3-diene.
There are two phases in the conventional synthesis of chloroprene. Acetylene is first dimerized to vinylacetylene in a reaction tower at 80 °C in an aqueous hydro-chloric acid solution of Cu2Cl2 and NH4Cl.
Water evaporation regulates the significant evolution of heat. The conversion of C2H2 is around 18%. The major by-product is divinylacetylene, and the selectivity to vinylacetylene can reach 90%. In the second stage HCl is added to vinylacetylene, forming chloropropene.

The correct answer is option (C) Cu2Cl2 + NH4Cl.
Additional Information: A catalyst is a substance that, without being consumed by the process, speeds up a chemical reaction or lowers the temperature or pressure required to initiate one. The addition of a catalyst to a reaction is known as catalysis. By lowering the activation energy, the energy barrier that must be cleared for a chemical reaction to take place, catalysts increase the efficiency of a reaction. There will be two steps in the conversion of acetylene to chloroprene. A triple bond and two carbon atoms are both present in the organic molecule acetylene. Four carbon atoms make up the chemical chloroprene, which also contains a chlorine atom in the second position. There are double bonds in the first and third positions.
Note: A triple bond and two carbon atoms are both present in the organic molecule acetylene. Four carbon atoms make up the chemical chloroprene, which also contains a chlorine atom in the second position. There will be two processes required to transform acetylene into chloroprene.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




