
Carry the following conversions
1).Propene to acetone
2).Bromobenzene to 1-phenyl ethanol
Answer
510.9k+ views
Hint: There are ways to convert an alkene to ketone. The reaction has two steps involving hydration followed by hydration. Hydration involves addition of water molecules which will lead to certain products. Bromobenzene is a structure having a benzene ring with a bromine atom.
Complete answer:
1).Propene is converted to acetone or propanone by following two steps step 1 involves hydration and step 2 involves oxidation.
Firstly an alkene is converted into corresponding alcohol. Propene on hydration gives isopropyl alcohol. The first step includes protonation of propene which leads to the formation of carbocation. Then the water molecules act as a nucleophile which results in the formation of oxonium ions and finally the oxonium ions are deprotonated by a base.
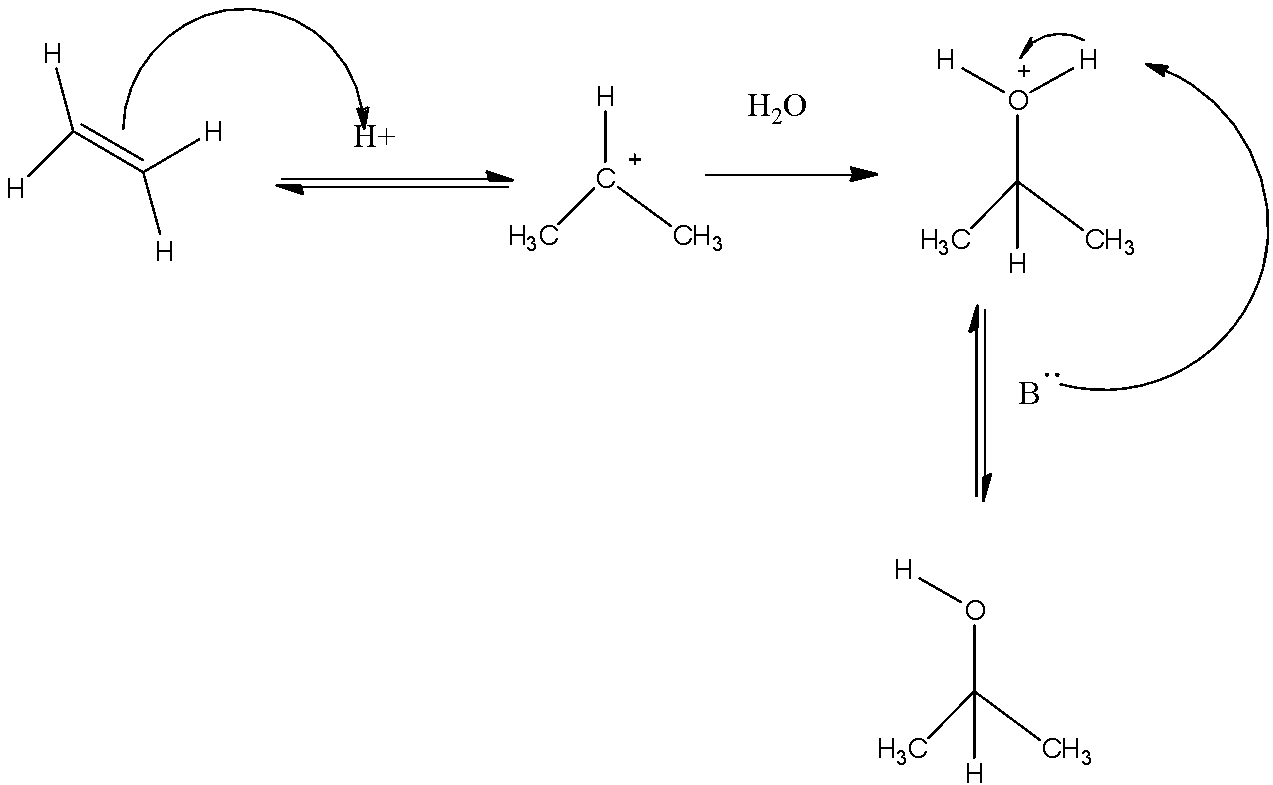
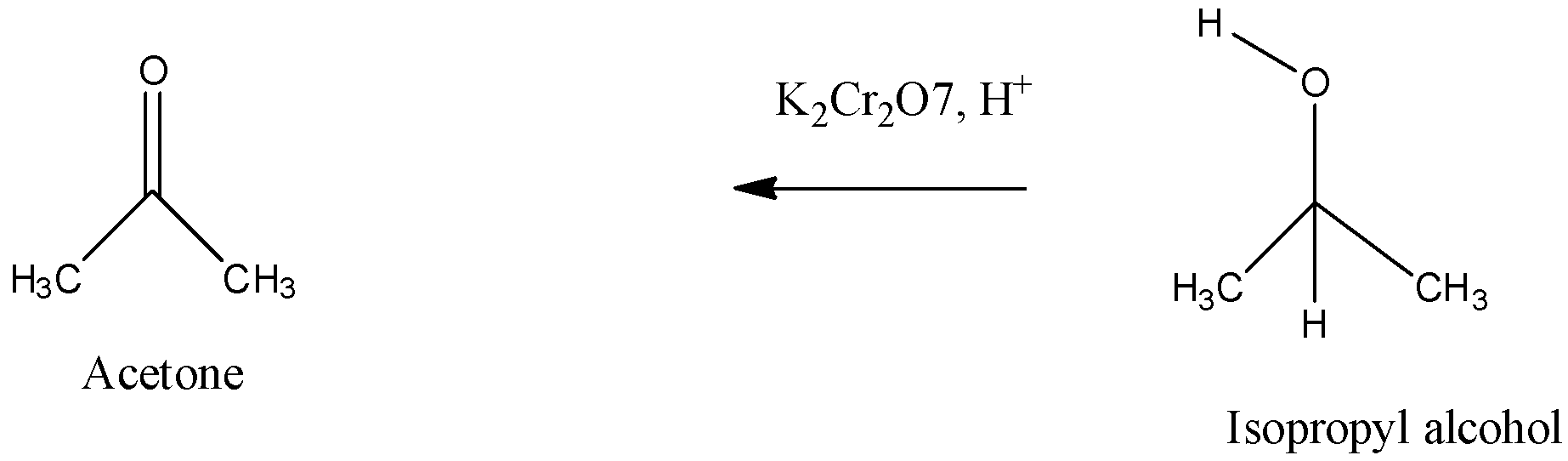
The second step involves the oxidation in which isopropyl alcohol is oxidized using an oxidizing agent, we can use potassium dichromate for the oxidation of alcohol to ketone.
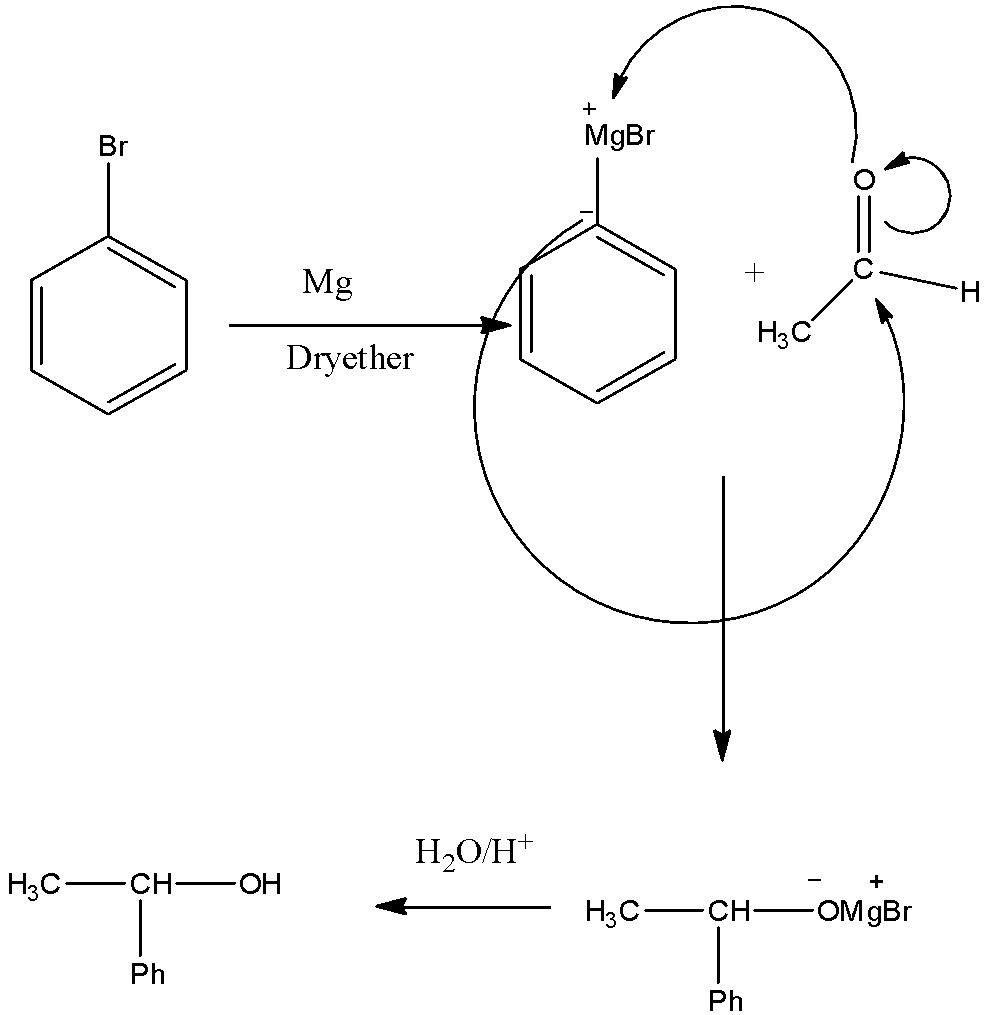
2).Bromobenzene is firstly converted into Grignard salt. This reaction is carried out in the presence of dry ether and Mg. This Grignard salt is reacted with ethanal which is then hydrolysis to give the product as 1-phenyl ethanol.
Note:
We can use different oxidizing agents like in place of potassium dichromate we can use PCC in acidic medium, it is a milder acid for chromic acid. On hydrolysis the ions are converted into hydroxyl salt. Isopropyl alcohol is a secondary alcohol. On oxidation secondary alcohol is converted into ketones.
Complete answer:
1).Propene is converted to acetone or propanone by following two steps step 1 involves hydration and step 2 involves oxidation.
Firstly an alkene is converted into corresponding alcohol. Propene on hydration gives isopropyl alcohol. The first step includes protonation of propene which leads to the formation of carbocation. Then the water molecules act as a nucleophile which results in the formation of oxonium ions and finally the oxonium ions are deprotonated by a base.

The second step involves the oxidation in which isopropyl alcohol is oxidized using an oxidizing agent, we can use potassium dichromate for the oxidation of alcohol to ketone.

2).Bromobenzene is firstly converted into Grignard salt. This reaction is carried out in the presence of dry ether and Mg. This Grignard salt is reacted with ethanal which is then hydrolysis to give the product as 1-phenyl ethanol.

Note:
We can use different oxidizing agents like in place of potassium dichromate we can use PCC in acidic medium, it is a milder acid for chromic acid. On hydrolysis the ions are converted into hydroxyl salt. Isopropyl alcohol is a secondary alcohol. On oxidation secondary alcohol is converted into ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




