
Carbocyclic compounds among the following:
A. Cyclohexane
B. Naphthalene
C. Pyrrole
D. Cyclopropene
Answer
531.9k+ views
Hint: The compounds (hydrocarbons) which are having a ring like structure and contain only carbon atoms in the ring are called carbocyclic compounds. If is there any hetero atom is present in the cyclic ring then the ring is called heterocyclic ring.
Complete answer:
- In the question it is given to identify the carbocyclic compound among the given options.
- Before going to talk about the given options we should know about the structure of the compound.
- The structures of the compounds which are present in the given options are as follows.
- The structure of cyclohexane is as follows.
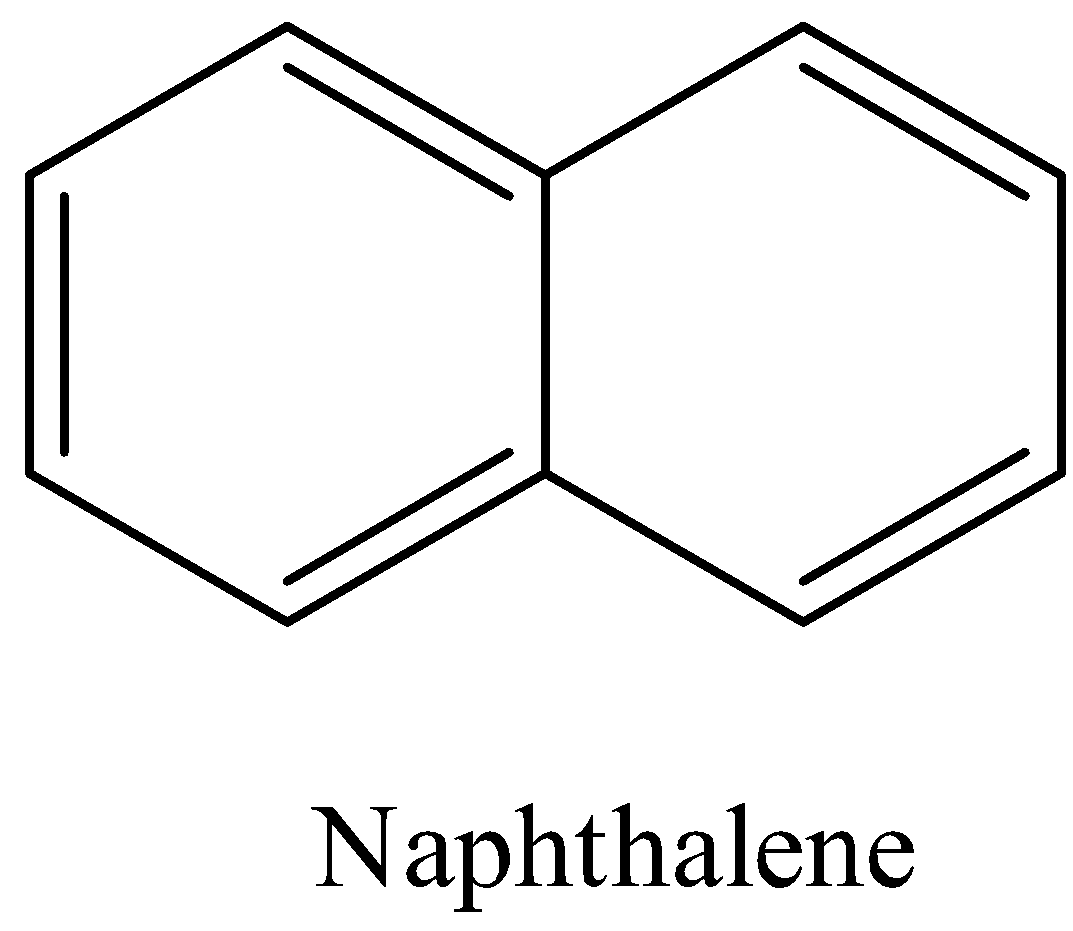
- Coming to the given option B, Naphthalene.
- The structure of the Naphthalene is as follows.
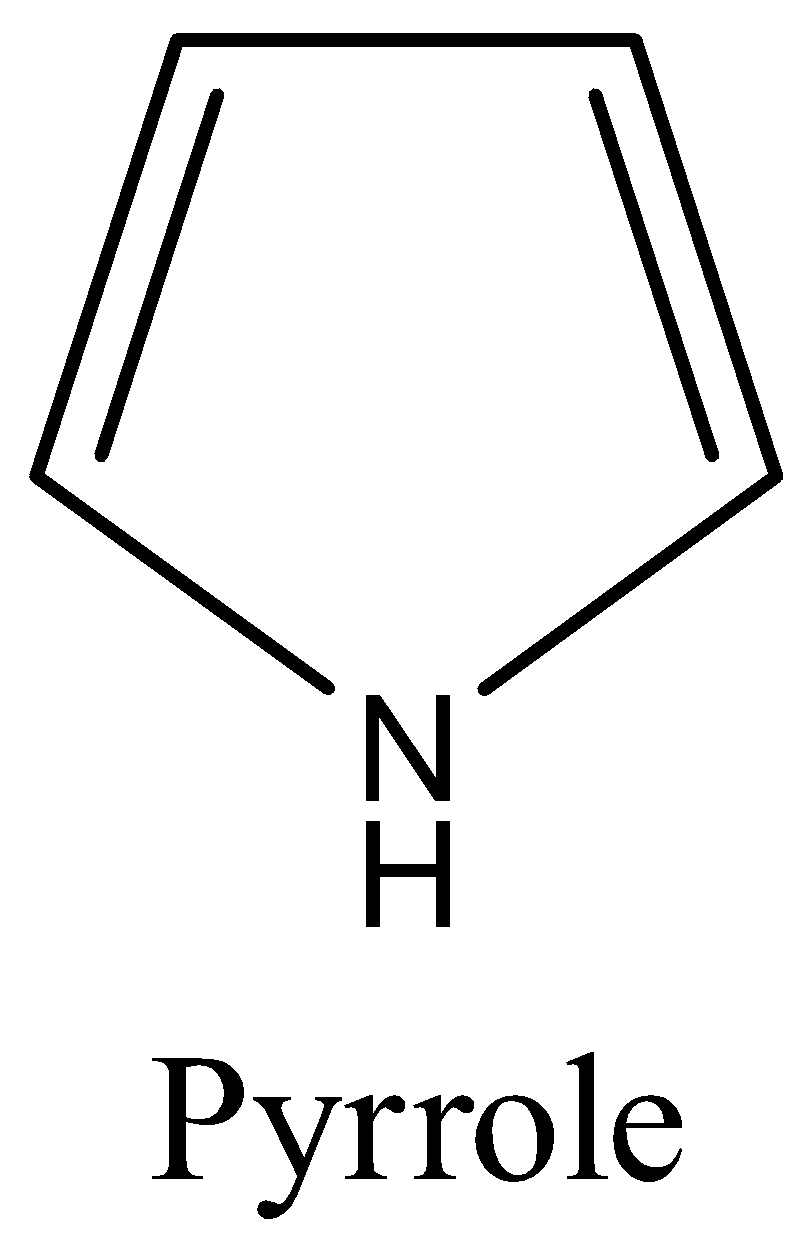
- Coming to the given option C, Pyrrole.
- The structure of the pyrrole ring is as follows.
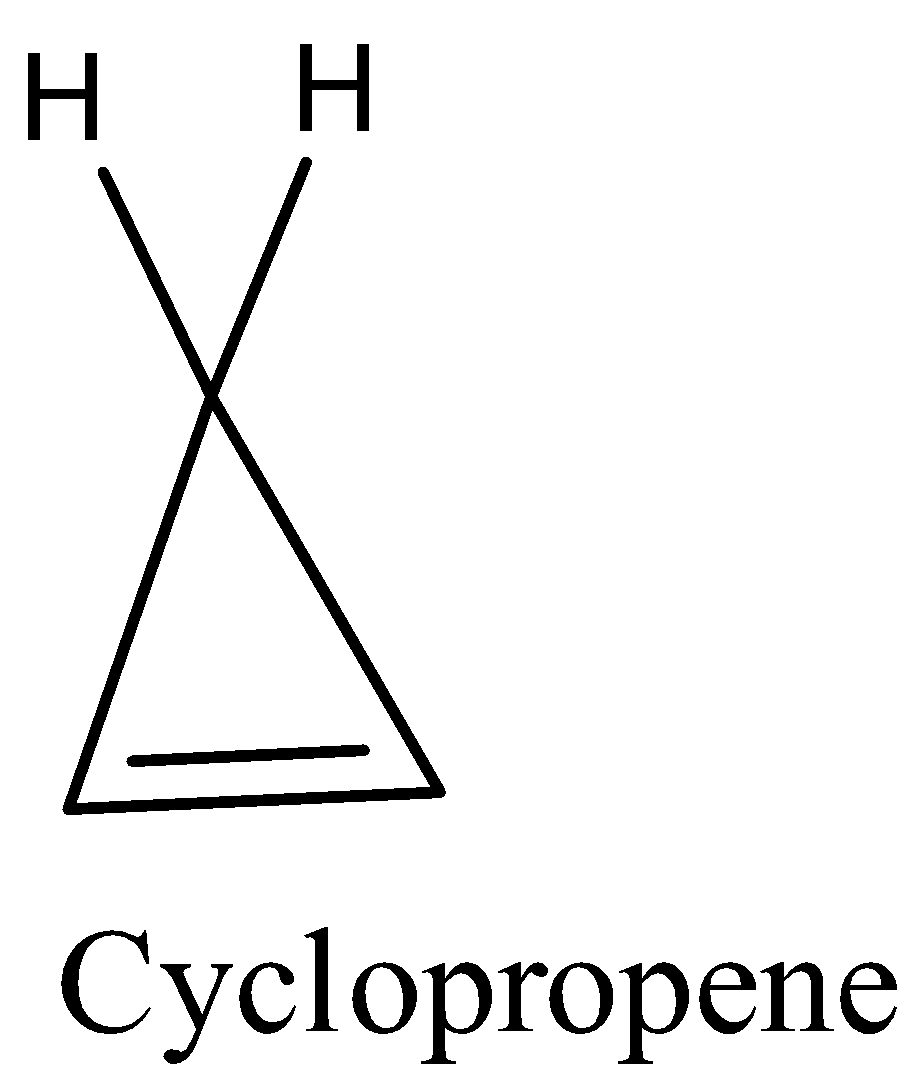
- Coming to the option D, cyclopropene.
- The structure of cyclopropene is as follows.
- Among the given only one ring is there which not a carbocyclic compound is.
- The compound which does not contain a carbocyclic ring among the given options is C.
- Because in the pyrrole ring there is a involvement of nitrogen atom (hetero atom) in the ring.
- Therefore the pyrrole Is not a carbocyclic compound.
So, the correct options are A, B and D.
Note:
If there is no heteroatom in the cyclic ring structure and the cyclic ring is called carbocyclic rings. Carbocyclic rings have high stability when compared to hetero atoms containing cyclic ring.
Complete answer:
- In the question it is given to identify the carbocyclic compound among the given options.
- Before going to talk about the given options we should know about the structure of the compound.
- The structures of the compounds which are present in the given options are as follows.
- The structure of cyclohexane is as follows.
- Coming to the given option B, Naphthalene.
- The structure of the Naphthalene is as follows.

- Coming to the given option C, Pyrrole.
- The structure of the pyrrole ring is as follows.

- Coming to the option D, cyclopropene.
- The structure of cyclopropene is as follows.

- Among the given only one ring is there which not a carbocyclic compound is.
- The compound which does not contain a carbocyclic ring among the given options is C.
- Because in the pyrrole ring there is a involvement of nitrogen atom (hetero atom) in the ring.
- Therefore the pyrrole Is not a carbocyclic compound.
So, the correct options are A, B and D.
Note:
If there is no heteroatom in the cyclic ring structure and the cyclic ring is called carbocyclic rings. Carbocyclic rings have high stability when compared to hetero atoms containing cyclic ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




