
Cannizzaro reaction is used mostly in aldehydes with _____ $\alpha $H-atoms.
Answer
576.9k+ views
Hint: This reaction, unlike aldol condensation, does not involve the $\alpha $H-atoms. The reactants are aldehydes only and not any other carbonyl compound. They undergo a disproportionation reaction to give products.
Complete step-by-step answer:
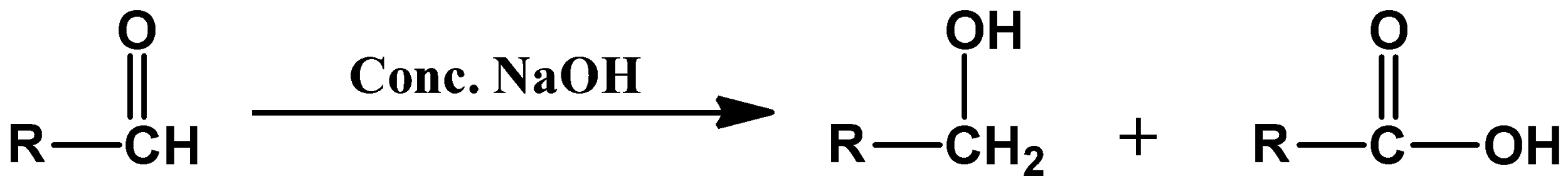
Cannizzaro reaction takes place in the presence of concentrated alkali such as sodium hydroxide. In this reaction, a molecule of aldehyde undergoes self-oxidation and self-reduction to form an acid and alcohol respectively. And therefore we call it a disproportionation reaction. You should always remember that this is only possible if the aldehyde used in the reaction does not have any alpha-hydrogen atoms. The general formula of this reaction is demonstrated as follows:
Additional information:
- $\alpha $H-atoms are the hydrogen atoms which are attached to the carbon atom directly attached to functional groups. And by functional group we mean to say any functional group known in organic chemistry. These $\alpha $H-atoms are acidic when the functional groups are electron withdrawing in nature due to inductive effect or resonance, as for example an aldehyde or carboxylic acid. Below we show the position of $\alpha $H-atoms in case of an aldehyde for better understanding of their relative position in accordance with the functional group.
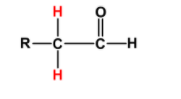
The hydrogen atoms marked in red are the $\alpha $H-atoms we are talking about.
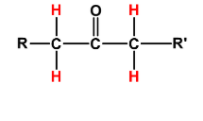
Ketones have $\alpha $H-atoms on both of their sides. You can see it below in red colour:
The acidity of these hydrogen atoms increase with the increase in electron withdrawing character of the functional groups associated with them.
Note: In an aldehyde group, the hydrogen atom already present is not considered as an alpha-hydrogen. If any carbon atom is attached to the aldehyde carbon, then the hydrogen atoms attached to the former are termed as $\alpha $H-atoms.
Complete step-by-step answer:
Cannizzaro reaction takes place in the presence of concentrated alkali such as sodium hydroxide. In this reaction, a molecule of aldehyde undergoes self-oxidation and self-reduction to form an acid and alcohol respectively. And therefore we call it a disproportionation reaction. You should always remember that this is only possible if the aldehyde used in the reaction does not have any alpha-hydrogen atoms. The general formula of this reaction is demonstrated as follows:

Additional information:
- $\alpha $H-atoms are the hydrogen atoms which are attached to the carbon atom directly attached to functional groups. And by functional group we mean to say any functional group known in organic chemistry. These $\alpha $H-atoms are acidic when the functional groups are electron withdrawing in nature due to inductive effect or resonance, as for example an aldehyde or carboxylic acid. Below we show the position of $\alpha $H-atoms in case of an aldehyde for better understanding of their relative position in accordance with the functional group.

The hydrogen atoms marked in red are the $\alpha $H-atoms we are talking about.
Ketones have $\alpha $H-atoms on both of their sides. You can see it below in red colour:

The acidity of these hydrogen atoms increase with the increase in electron withdrawing character of the functional groups associated with them.
Note: In an aldehyde group, the hydrogen atom already present is not considered as an alpha-hydrogen. If any carbon atom is attached to the aldehyde carbon, then the hydrogen atoms attached to the former are termed as $\alpha $H-atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




