
Calculate $x + y + z$ for ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$ acid, where x is the number of lone pair, y is the number of $\sigma $ bonds and z is the number of $\pi $
bonds.
A. 5
B. 14
C.13
D. 12
Answer
585.3k+ views
Hint: We know that, the molecular formula of phosphorous acid is ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$. This acid is a colourless oxyacid of phosphorus. It is a white colored powder and can be produced by the combustion of phosphorus.
Complete step by step answer: We know that, $\sigma $bonds are the single bonds present in a compound
One double bond consist of one $\sigma $ bond and one $\pi $ bond
One triple bond consists of one $\sigma $bond and two $\pi $ bonds
Now, we draw the structure of ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$
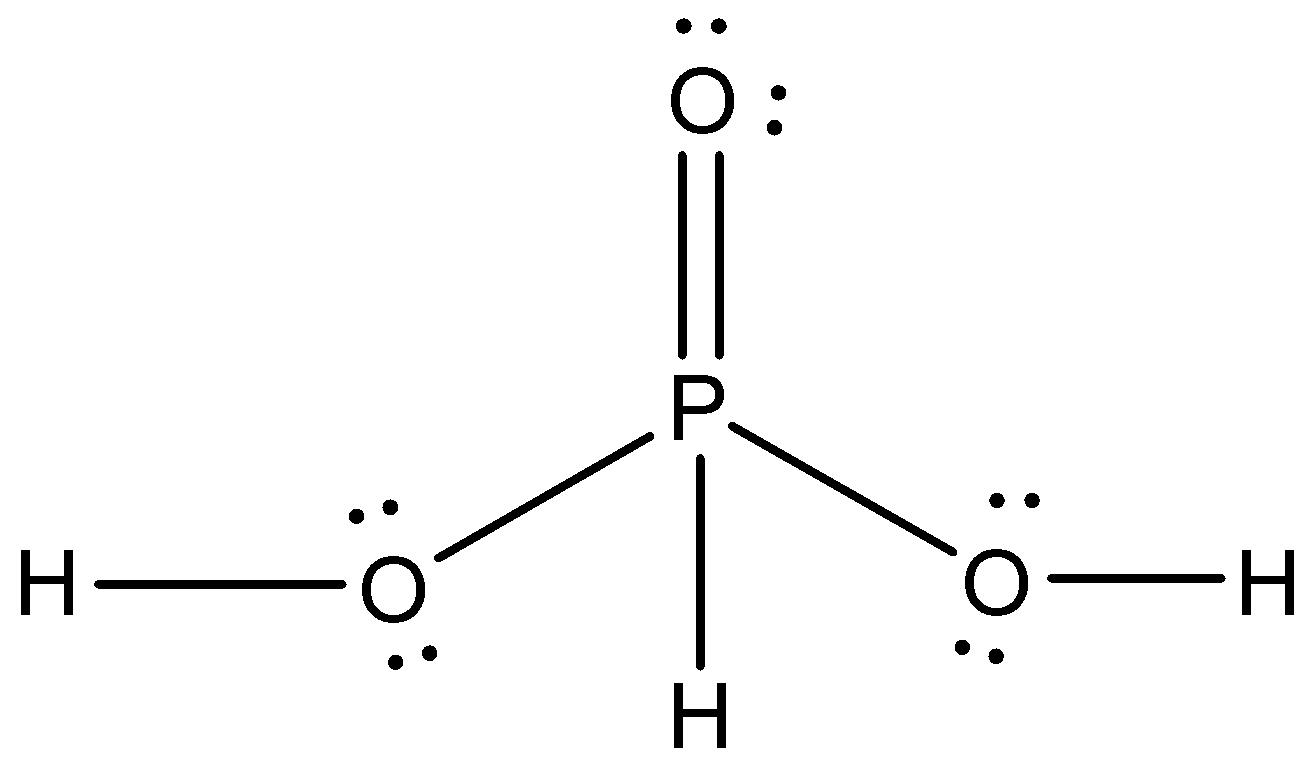
In ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$,
-5 single bonds present, that means, 5 $\sigma $bonds
-1 double bonds present, that means, 1 $\sigma $ bond and 1 $\pi $ bond
-6 lone pairs present
So, the total number of $\sigma $ bonds in phosphorus acid is 6, total number of $\pi $
bonds in the compound is one and the total number of lone pairs in the compound is 6.
So, the summation of $\sigma $ bond (X), $\pi $ bond (y) and lone pair (z) is=$6 + 1 + 6 = 13$
Hence, the correct option is C.
Additional Information:
Phosphorous acid $\left( {{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}} \right)$ possesses reducing properties and tends to convert to phosphoric acid. On heating dry phosphorus acid, disproportionation of phosphorus acid takes place which leads to the formation of phosphoric acid and phosphine.
${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} \to {\rm{P}}{{\rm{H}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{4}}}$
The reaction of phosphorus acid with base (NaOH) produces water and sodium Phosphate.
\[{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{\rm{NaOH}} \to {\rm{N}}{{\rm{a}}_3}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{2}}}{\rm{O}}\]
Note: Phosphoric acid ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$ is a monobasic acid as it has two OH groups in the compound. Its use in production of phosphonate PVC stabilizer, hydroxyethane diphosphonic acid is very important. As it is a strong reducing agent, it is used in many chemical reactions. The raw materials of synthetic fibres are also prepared from phosphoric acid.
Complete step by step answer: We know that, $\sigma $bonds are the single bonds present in a compound
One double bond consist of one $\sigma $ bond and one $\pi $ bond
One triple bond consists of one $\sigma $bond and two $\pi $ bonds
Now, we draw the structure of ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$

In ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$,
-5 single bonds present, that means, 5 $\sigma $bonds
-1 double bonds present, that means, 1 $\sigma $ bond and 1 $\pi $ bond
-6 lone pairs present
So, the total number of $\sigma $ bonds in phosphorus acid is 6, total number of $\pi $
bonds in the compound is one and the total number of lone pairs in the compound is 6.
So, the summation of $\sigma $ bond (X), $\pi $ bond (y) and lone pair (z) is=$6 + 1 + 6 = 13$
Hence, the correct option is C.
Additional Information:
Phosphorous acid $\left( {{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}} \right)$ possesses reducing properties and tends to convert to phosphoric acid. On heating dry phosphorus acid, disproportionation of phosphorus acid takes place which leads to the formation of phosphoric acid and phosphine.
${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} \to {\rm{P}}{{\rm{H}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{4}}}$
The reaction of phosphorus acid with base (NaOH) produces water and sodium Phosphate.
\[{{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{\rm{NaOH}} \to {\rm{N}}{{\rm{a}}_3}{\rm{P}}{{\rm{O}}_{\rm{3}}} + 3{{\rm{H}}_{\rm{2}}}{\rm{O}}\]
Note: Phosphoric acid ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$ is a monobasic acid as it has two OH groups in the compound. Its use in production of phosphonate PVC stabilizer, hydroxyethane diphosphonic acid is very important. As it is a strong reducing agent, it is used in many chemical reactions. The raw materials of synthetic fibres are also prepared from phosphoric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




