
Calculate the number of atoms per unit cell of FCC and BCC crystal structure.
Answer
615k+ views
Hint: Solve the given question using basic knowledge of solid state systems. Solving the question by using diagrams of unit cells would be beneficial.
Complete step by step answer:
This is the structure of a simple or primitive cubic unit cell-
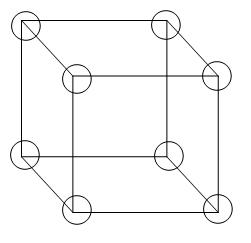
From the diagram we can see that 8 atoms are arranged in all the corners of the cube, therefore, the contribution per unit cell will be 1 atom,
\[\because 8\text{x}\dfrac{1}{8}=1\] atom or molecule
This is the structure of a body-centred unit cell-
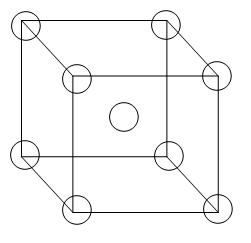
From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. Therefore, the contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred)
=2
This is the structure of a face-centred unit cell-
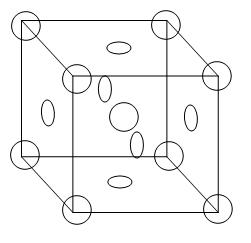
From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. In addition to this, there are atoms on each face of the cube. Therefore, the contribution of atoms on the cube = \[6\text{x}\dfrac{1}{2}=3\]
Therefore, total contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred) + 3 (face centred)
= 4.
Therefore, the answer is –
The number of atoms per unit cell of FCC crystal structure = 4 and,
The number of atoms per unit cell of BCC crystal structure = 2.
Note: Unit cell is the simplest unit of a complete crystal lattice. It is the most uniform unit used for analysis of the structure.
Complete step by step answer:
This is the structure of a simple or primitive cubic unit cell-

From the diagram we can see that 8 atoms are arranged in all the corners of the cube, therefore, the contribution per unit cell will be 1 atom,
\[\because 8\text{x}\dfrac{1}{8}=1\] atom or molecule
This is the structure of a body-centred unit cell-

From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. Therefore, the contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred)
=2
This is the structure of a face-centred unit cell-

From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. In addition to this, there are atoms on each face of the cube. Therefore, the contribution of atoms on the cube = \[6\text{x}\dfrac{1}{2}=3\]
Therefore, total contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred) + 3 (face centred)
= 4.
Therefore, the answer is –
The number of atoms per unit cell of FCC crystal structure = 4 and,
The number of atoms per unit cell of BCC crystal structure = 2.
Note: Unit cell is the simplest unit of a complete crystal lattice. It is the most uniform unit used for analysis of the structure.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




