
Calculate the mass of three moles of ethane.
Answer
524.4k+ views
Hint: In order to solve this question, one must be familiar with the mole concept. Ethane is made up of carbon and hydrogen. Their respective standard atomic weights, when added in their respective proportions (quantities), will provide the mass of one mole of ethane. This will give us the molar mass or molecular weight of ethane.
Complete answer:
The structure of the given compound, ethane is
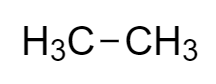
Ethane is an inflammable alkane, having the chemical formula ${C_2}{H_6}$ .
Now, as carbon and hydrogen are the respective constituents of ethane, we need to calculate their individual mass in one mole of ethane.
Thus, as the atomic weight of carbon $ = \,12$ and as there are two carbon atoms present in the compound, the amount of carbon in one mole of ethane $ = \,12\, \times \,2\, = \,24$ grams.
Similarly, we now compute for hydrogen, whose atomic weight $ = \,1$ , and as there are six hydrogen atoms present in ethane, the amount of hydrogen present in one mole of ethane $ = \,1\, \times \,6\, = \,6$ grams.
Therefore, the mass of one mole of ethane (molar mass) $ = \,24\, + \,6\, = \,30$ grams.
For three moles of ethane, we multiply its molar mass by three (by applying the unitary method), and we get $ = \,30\, \times \,3\, = \,90$ grams.
Thus, the mass of three moles of ethane is $90$ grams.
Note:
For solving this question, one must be aware of the concept of atomic weights. The mass of a compound is the sum of standard atomic masses of its constituent atoms in their respective proportions or quantities. The molar mass of a compound, expressed in grams, is the mass of $6.022\, \times \,{10^{23}}$ particles of that substance.
Complete answer:
The structure of the given compound, ethane is
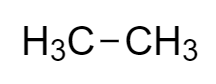
Ethane is an inflammable alkane, having the chemical formula ${C_2}{H_6}$ .
Now, as carbon and hydrogen are the respective constituents of ethane, we need to calculate their individual mass in one mole of ethane.
Thus, as the atomic weight of carbon $ = \,12$ and as there are two carbon atoms present in the compound, the amount of carbon in one mole of ethane $ = \,12\, \times \,2\, = \,24$ grams.
Similarly, we now compute for hydrogen, whose atomic weight $ = \,1$ , and as there are six hydrogen atoms present in ethane, the amount of hydrogen present in one mole of ethane $ = \,1\, \times \,6\, = \,6$ grams.
Therefore, the mass of one mole of ethane (molar mass) $ = \,24\, + \,6\, = \,30$ grams.
For three moles of ethane, we multiply its molar mass by three (by applying the unitary method), and we get $ = \,30\, \times \,3\, = \,90$ grams.
Thus, the mass of three moles of ethane is $90$ grams.
Note:
For solving this question, one must be aware of the concept of atomic weights. The mass of a compound is the sum of standard atomic masses of its constituent atoms in their respective proportions or quantities. The molar mass of a compound, expressed in grams, is the mass of $6.022\, \times \,{10^{23}}$ particles of that substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




