
Calculate the lattice energy of a salt \[MX(s)\] from the date given below:
Heat of formation of \[MX\left( {\Delta H} \right)\; = - 550\;kJ/mol\]
Heat of sublimation of \[M\left( S \right) = 80\;kJ/mol\]
Heat of dissociation of \[{X_2}\left( D \right) = 155\;kJ/mol\]
Ionization energy of \[M\left( {IE} \right) = 347\;kJ/mol\]
Electron affinity of \[X\left( {EA} \right) = - 343\;kJ/mol\]
A. \[ - 835KJ/mol\]
B. \[ - 938.5{\text{ }}kJ/mol\]
C. \[ - 711.5{\text{ }}kJ/mol\]
D. \[ - 638.5{\text{ }}kJ/mol\]
Answer
567k+ views
Hint:Born Haber cycle is mainly used to calculate the lattice energy. It also involves steps such as sublimation energy \[\left( {\Delta {H_{sub}}\;} \right),\] dissociation energy \[\left( D \right),\] ionisation energy\[\left( I \right),\] electron affinity\[\left( E \right)\] and heat of formation of crystal \[\left( H \right).\] Represented in the form of
\[\Delta {\text{ }}H{\;_{f{\;^0}}}\; = {\text{ }}\Delta H\;sub\; + {\text{ }}\dfrac{D}{2} + {\text{ }}IE{\text{ }} + {\text{ }}EA\; + U\]
Complete step by step answer:
Born Haber cycle is a cycle of enthalpy change of process and The energy terms involved in building a crystal lattice\[\left( {{\text{ }}MX} \right)\] such as -
Step\[\;1\] - convert solid \[\left( {{\text{ }}M} \right)\] to gaseous \[\left( {{\text{ }}M} \right)\]is called enthalpy of sublimation \[\left( {\Delta H\;sub} \right).\]
Step \[2\] - convert gaseous\[\left( X \right)\] molecule to atoms is called enthalpy of dissociation\[\left( D \right).\]
Step \[3\] - Conversion of gaseous \[\left( {{\text{ }}M} \right)\] atom into \[\left( {M{\text{ }}ion} \right)\] in gaseous state is called ionisation energy .
Step \[4\] - Conversion of gaseous \[\left( X \right)\] atom into gaseous \[X{\text{ }}\;ion\] is known as electron gain enthalpy and represented by \[{E_A}\;.\]
Step \[5\] - The amount of energy released when one mole of solid crystalline compound is obtained from gaseous ions is called lattice energy\[\left( U \right)\]
\[M\left( s \right) + {\text{ }}\dfrac{1}{2}X\;\left( g \right) \to MX\left( s \right)\]
The Born-Haber Cycle can be reduced to a single equation:
Heat of formation= Heat of atomization + Dissociation energy+ (sum of Ionization energies) + (sum of Electron affinities)+ Lattice energy
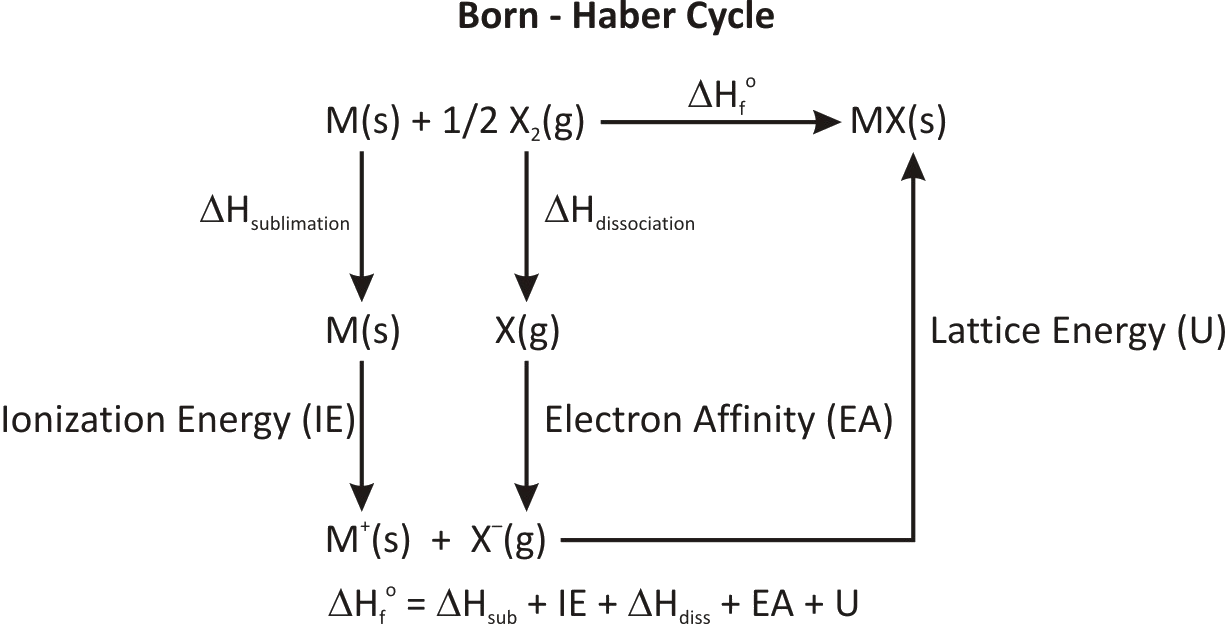
The enthalpies are represented in figure.
These steps are represented as -
\[\Delta {\text{ }}H{\;_f}^0\; = {\text{ }}\Delta H{\;_{sub}}\; + {\text{ }}D/2{\text{ }} + {\text{ }}IE{\text{ }} + {\text{ }}{E_A}\; + U\]
Now, Putting values as -
\[\Delta {H_f}\; = \;\;\;S{\text{ }} + {\text{ }}0.5D{\text{ }} + {\text{ }}IE{\text{ }} + {\text{ }}EA{\text{ }} + {\text{ }}U\]
\[ - 550{\text{ }} = \;\;80 + \dfrac{{155}}{2} + 347 - 343 + U\]
\[\;\;U = - 711.5kJ/mol\]
lattice energy of salt \[ = \; - 711.5\;kJ/mol.\]
Option (c ) is correct.
Note:Born Haber process is a method that allows us to observe and analyze energies in a reaction. It mainly helps in describing the formation of ionic compounds from different elements. Born Haber cycle is a process that leads to the formation of a solid crystalline ionic compound from the elemental atoms in their standard state and of the enthalpy of formation of the solid compound such that the net enthalpy becomes zero.
\[\Delta {\text{ }}H{\;_{f{\;^0}}}\; = {\text{ }}\Delta H\;sub\; + {\text{ }}\dfrac{D}{2} + {\text{ }}IE{\text{ }} + {\text{ }}EA\; + U\]
Complete step by step answer:
Born Haber cycle is a cycle of enthalpy change of process and The energy terms involved in building a crystal lattice\[\left( {{\text{ }}MX} \right)\] such as -
Step\[\;1\] - convert solid \[\left( {{\text{ }}M} \right)\] to gaseous \[\left( {{\text{ }}M} \right)\]is called enthalpy of sublimation \[\left( {\Delta H\;sub} \right).\]
Step \[2\] - convert gaseous\[\left( X \right)\] molecule to atoms is called enthalpy of dissociation\[\left( D \right).\]
Step \[3\] - Conversion of gaseous \[\left( {{\text{ }}M} \right)\] atom into \[\left( {M{\text{ }}ion} \right)\] in gaseous state is called ionisation energy .
Step \[4\] - Conversion of gaseous \[\left( X \right)\] atom into gaseous \[X{\text{ }}\;ion\] is known as electron gain enthalpy and represented by \[{E_A}\;.\]
Step \[5\] - The amount of energy released when one mole of solid crystalline compound is obtained from gaseous ions is called lattice energy\[\left( U \right)\]
\[M\left( s \right) + {\text{ }}\dfrac{1}{2}X\;\left( g \right) \to MX\left( s \right)\]
The Born-Haber Cycle can be reduced to a single equation:
Heat of formation= Heat of atomization + Dissociation energy+ (sum of Ionization energies) + (sum of Electron affinities)+ Lattice energy

The enthalpies are represented in figure.
These steps are represented as -
\[\Delta {\text{ }}H{\;_f}^0\; = {\text{ }}\Delta H{\;_{sub}}\; + {\text{ }}D/2{\text{ }} + {\text{ }}IE{\text{ }} + {\text{ }}{E_A}\; + U\]
Now, Putting values as -
\[\Delta {H_f}\; = \;\;\;S{\text{ }} + {\text{ }}0.5D{\text{ }} + {\text{ }}IE{\text{ }} + {\text{ }}EA{\text{ }} + {\text{ }}U\]
\[ - 550{\text{ }} = \;\;80 + \dfrac{{155}}{2} + 347 - 343 + U\]
\[\;\;U = - 711.5kJ/mol\]
lattice energy of salt \[ = \; - 711.5\;kJ/mol.\]
Option (c ) is correct.
Note:Born Haber process is a method that allows us to observe and analyze energies in a reaction. It mainly helps in describing the formation of ionic compounds from different elements. Born Haber cycle is a process that leads to the formation of a solid crystalline ionic compound from the elemental atoms in their standard state and of the enthalpy of formation of the solid compound such that the net enthalpy becomes zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




