
Calculate the degree of ionization of 0.05 M acetic acid if its ${{p}^{Ka}}$ value is 4.74. How is the degree of association affected when its solution is (a) 0.01 M and (b) 0.1 M in hydrochloric acid?
A. (a) Will decrease
(b) Will decrease
B. (a) Will decrease
(b) Will increase
C. (a) Will increase
(b) Will decrease
D. (a) Will increase
(b) Will increase
Answer
558.6k+ views
Hint: Write the equation for ionic dissociation of acetic acid. Then, using the value of Ka and concentration, calculate the value of dissociation constant. Use that value to check the effect of adding hydrochloric acid on the degree of dissociation.
Complete step by step answer:
Acetic acid dissociates as –
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
According to the question, we’ve been given a degree of ionization and pKa values of acetic acid.
Given,
Concentration, c = 0.05 M
${{p}^{Ka}}$ = 4.74
${{p}^{Ka}}$= -log (Ka)
Ka = $1.82\times {{10}^{-5}}$
We can relate Ka to degree of dissociation as-
\[\alpha =\sqrt{\dfrac{{{K}_{a}}}{c}}\]
\[\Rightarrow \alpha =\sqrt{\dfrac{1.82\times {{10}^{-5}}}{5\times {{10}^{-2}}}}=1.908\times {{10}^{-2}}\]
- Degree of dissociation is also equal to the ratio of amount of acid dissociated to the amount of acid taken.
If HCl is added to the solution, the concentration of ions will increase. Hence, the equilibrium will shift back i.e. dissociation of acetic acid will decrease.
- Let us discuss the two cases –
(Let the amount of acetic acid after dissociation = x)
- Case 1: 0.01 M HCl
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
${{K}_{a}}=\dfrac{\left[ C{{H}_{3}}CO{{O}^{-}} \right]\left[ {{H}^{+}} \right]}{\left[ C{{H}_{3}}COOH \right]}$
\[\Rightarrow 1.82\times {{10}^{-5}}=\dfrac{\left[ 0.01 \right]\left[ x \right]}{\left[ 0.05 \right]}\]
\[\Rightarrow x=\left( 1.82\times {{10}^{-3}} \right)\left( 0.05 \right)\]
- Now, since the degree of dissociation is the ratio of amount of acid dissociates to the amount of acid taken, we have
\[\alpha =\dfrac{\left( 1.82\times {{10}^{-3}} \right)\left( 0.05 \right)}{\left( 0.05 \right)}=1.82\times {{10}^{-3}}\]
- Case 2: 0.1 M HCl (replace 0.01 by 0.1)
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
${{K}_{a}}=\dfrac{\left[ C{{H}_{3}}CO{{O}^{-}} \right]\left[ {{H}^{+}} \right]}{\left[ C{{H}_{3}}COOH \right]}$
\[\Rightarrow 1.82\times {{10}^{-5}}=\dfrac{\left[ 0.1 \right]\left[ x \right]}{\left[ 0.05 \right]}\]
\[\Rightarrow x=\left( 1.82\times {{10}^{-4}} \right)\left( 0.05 \right)\]
Even here, the degree of dissociation is the ratio of amount of acid dissociates to the amount of acid taken, we have
\[\alpha =\dfrac{\left( 1.82\times {{10}^{-4}} \right)\left( 0.05 \right)}{\left( 0.05 \right)}=1.82\times {{10}^{-4}}\]
Therefore, the degree of dissociation without acetic acid is $1.908\times {{10}^{-2}}$.
Also, the degree of dissociation will decrease if HCl is added (as we can see through calculation in both cases). So the correct answer is “A”:
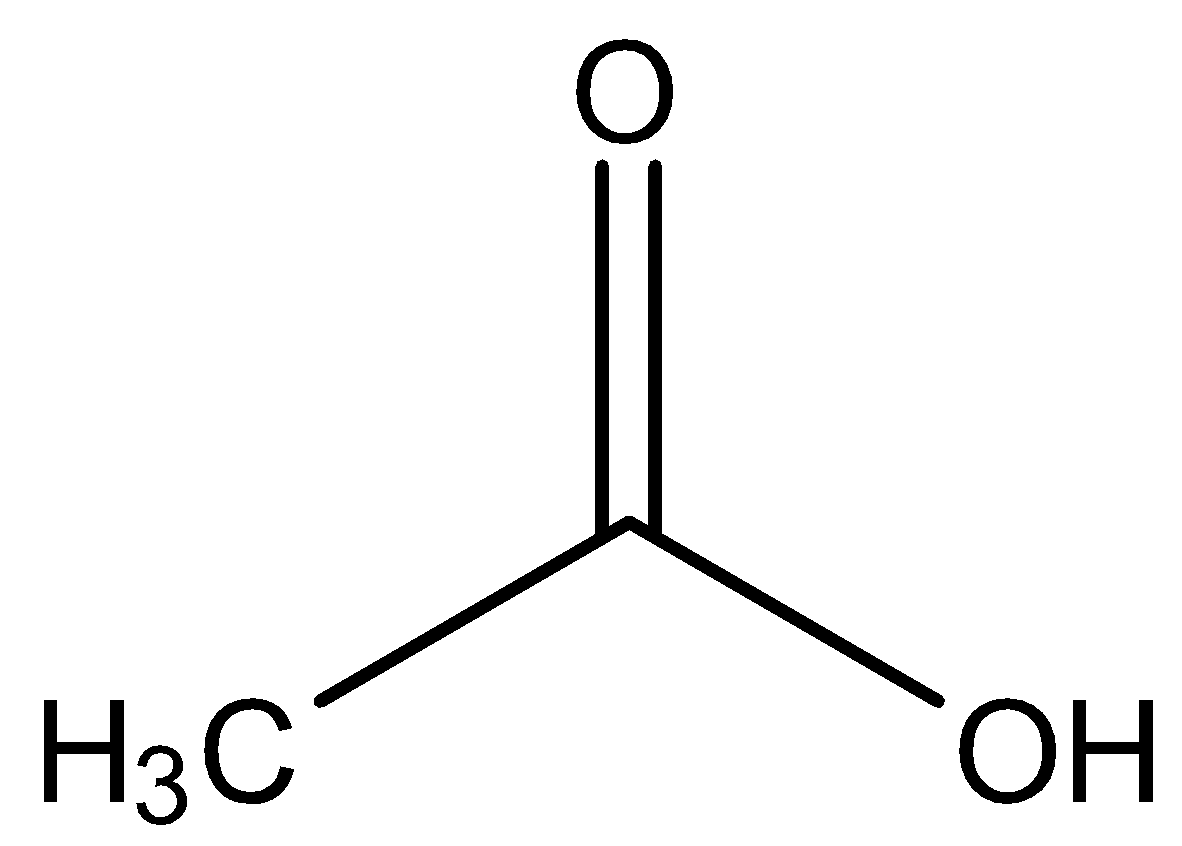
Additional Information: Acetic acid is also known as ethanoic acid. The structural formula of acetic acid is –
Note: Degree of dissociation can be defined as “the amount of solute dissociated into ions or radicals per mole”. It is the fraction of “original solute molecules that have dissociated”. It is represented by the Greek symbol $\alpha $.
Complete step by step answer:
Acetic acid dissociates as –
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
According to the question, we’ve been given a degree of ionization and pKa values of acetic acid.
Given,
Concentration, c = 0.05 M
${{p}^{Ka}}$ = 4.74
${{p}^{Ka}}$= -log (Ka)
Ka = $1.82\times {{10}^{-5}}$
We can relate Ka to degree of dissociation as-
\[\alpha =\sqrt{\dfrac{{{K}_{a}}}{c}}\]
\[\Rightarrow \alpha =\sqrt{\dfrac{1.82\times {{10}^{-5}}}{5\times {{10}^{-2}}}}=1.908\times {{10}^{-2}}\]
- Degree of dissociation is also equal to the ratio of amount of acid dissociated to the amount of acid taken.
If HCl is added to the solution, the concentration of ions will increase. Hence, the equilibrium will shift back i.e. dissociation of acetic acid will decrease.
- Let us discuss the two cases –
(Let the amount of acetic acid after dissociation = x)
- Case 1: 0.01 M HCl
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
| Initial conc. | 0.05 | 0 | 0 |
| After dissociation | 0.05 - x | 0.01 + x | x |
${{K}_{a}}=\dfrac{\left[ C{{H}_{3}}CO{{O}^{-}} \right]\left[ {{H}^{+}} \right]}{\left[ C{{H}_{3}}COOH \right]}$
\[\Rightarrow 1.82\times {{10}^{-5}}=\dfrac{\left[ 0.01 \right]\left[ x \right]}{\left[ 0.05 \right]}\]
\[\Rightarrow x=\left( 1.82\times {{10}^{-3}} \right)\left( 0.05 \right)\]
- Now, since the degree of dissociation is the ratio of amount of acid dissociates to the amount of acid taken, we have
\[\alpha =\dfrac{\left( 1.82\times {{10}^{-3}} \right)\left( 0.05 \right)}{\left( 0.05 \right)}=1.82\times {{10}^{-3}}\]
- Case 2: 0.1 M HCl (replace 0.01 by 0.1)
\[C{{H}_{3}}COOHC{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
| Initial conc. | 0.05 | 0 | 0 |
| After dissociation | 0.05 - x | 0.01 + x | x |
${{K}_{a}}=\dfrac{\left[ C{{H}_{3}}CO{{O}^{-}} \right]\left[ {{H}^{+}} \right]}{\left[ C{{H}_{3}}COOH \right]}$
\[\Rightarrow 1.82\times {{10}^{-5}}=\dfrac{\left[ 0.1 \right]\left[ x \right]}{\left[ 0.05 \right]}\]
\[\Rightarrow x=\left( 1.82\times {{10}^{-4}} \right)\left( 0.05 \right)\]
Even here, the degree of dissociation is the ratio of amount of acid dissociates to the amount of acid taken, we have
\[\alpha =\dfrac{\left( 1.82\times {{10}^{-4}} \right)\left( 0.05 \right)}{\left( 0.05 \right)}=1.82\times {{10}^{-4}}\]
Therefore, the degree of dissociation without acetic acid is $1.908\times {{10}^{-2}}$.
Also, the degree of dissociation will decrease if HCl is added (as we can see through calculation in both cases). So the correct answer is “A”:
Additional Information: Acetic acid is also known as ethanoic acid. The structural formula of acetic acid is –

Note: Degree of dissociation can be defined as “the amount of solute dissociated into ions or radicals per mole”. It is the fraction of “original solute molecules that have dissociated”. It is represented by the Greek symbol $\alpha $.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




