
\[{C_7}{H_{16}}\] has 9 isomers. How many of these isomers have quaternary carbons?
A.1
B.2
C.3
D.4
Answer
583.8k+ views
Hint: We can see from the structural formula that the parent carbon chain will have seven carbon atoms. This gives it a name heptane. Structural isomers are those molecules which have the same molecular formula but different bonding patterns such as branching. Therefore, we can arrange the carbon atoms of heptane to form different structural isomers.
Complete step-by-step answer:
Isomers are those molecules which have the same chemical formula but distinct and different structures. The bonding structure tells us the way atoms are bonded in the molecule. Heptane is an organic compound which comes under the category of alkanes having the general formula \[{C_n}{H_{2n + 2}}\] , here \[n = 7\] . Alkanes are those organic compounds which do not have double or triple bonds and so, they are saturated compounds.
We are provided with the molecular formula of heptane i.e. \[{C_7}{H_{16}}\] . The seven carbon atoms present in it are rearranged in nine distinct ways to form nine isomers. Let us see the arrangement of these seven carbon atoms and formation of nine isomers. The strategy is to start with the straight chain isomer or longest chain. Going to a six-carbon chain, a methyl group may be placed either on C2 or on C3 so as to give two isomers.
Starting with a five-carbon chain, either two methyl or an ethyl group must be added as a side chain for a total of seven carbons. The two methyl groups may be placed on the same C atom or on different carbons giving a total of four isomers but the ethyl group can be placed only on the central C atom. At last, go for a four-carbon chain and three methyl groups to form the ninth isomer.
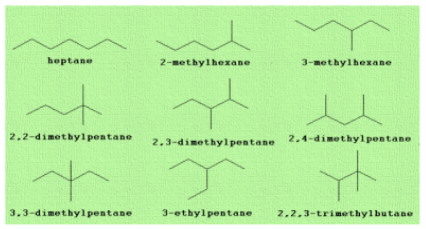
We know that quaternary carbon is a carbon atom which is bonded to 4 other atoms. So, from these nine structures we can see that out of these, three isomers have quaternary carbons. These are:
2,2-dimethylpentane, 3,3-dimethylpentane and 2,2,3-trimethylbutane.
Hence, the correct option is (C).
Note: We can also use the formula of calculating isomers for Alkanes i.e. \[2\left( {n - 4} \right) + 3\] . These are structural isomers where n is the number of carbon atoms. If we want to include stereoisomers also then in two of the structures, we will have one enantiomer each because they have one chiral centre each. Therefore, we will have 11 isomers total but 9 structural isomers.
Complete step-by-step answer:
Isomers are those molecules which have the same chemical formula but distinct and different structures. The bonding structure tells us the way atoms are bonded in the molecule. Heptane is an organic compound which comes under the category of alkanes having the general formula \[{C_n}{H_{2n + 2}}\] , here \[n = 7\] . Alkanes are those organic compounds which do not have double or triple bonds and so, they are saturated compounds.
We are provided with the molecular formula of heptane i.e. \[{C_7}{H_{16}}\] . The seven carbon atoms present in it are rearranged in nine distinct ways to form nine isomers. Let us see the arrangement of these seven carbon atoms and formation of nine isomers. The strategy is to start with the straight chain isomer or longest chain. Going to a six-carbon chain, a methyl group may be placed either on C2 or on C3 so as to give two isomers.
Starting with a five-carbon chain, either two methyl or an ethyl group must be added as a side chain for a total of seven carbons. The two methyl groups may be placed on the same C atom or on different carbons giving a total of four isomers but the ethyl group can be placed only on the central C atom. At last, go for a four-carbon chain and three methyl groups to form the ninth isomer.

We know that quaternary carbon is a carbon atom which is bonded to 4 other atoms. So, from these nine structures we can see that out of these, three isomers have quaternary carbons. These are:
2,2-dimethylpentane, 3,3-dimethylpentane and 2,2,3-trimethylbutane.
Hence, the correct option is (C).
Note: We can also use the formula of calculating isomers for Alkanes i.e. \[2\left( {n - 4} \right) + 3\] . These are structural isomers where n is the number of carbon atoms. If we want to include stereoisomers also then in two of the structures, we will have one enantiomer each because they have one chiral centre each. Therefore, we will have 11 isomers total but 9 structural isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




