
$ {C_6}{H_{12}}(A) $ on treatment with $ HCl $ produces a compound $ Y $ , which is optically inactive. What is the structure of $ A $ ?
Answer
547.5k+ views
Hint: An optically inactive compound, is one which does not show optical rotation. Optical activity of an organic compound refers to the property of an organic compound by virtue of which, when it is passed through their solutions, the plane polarized light (created by transmitting ordinary light via Nicol prism) rotates and the compounds are known as optically active compounds. Optically active compounds must possess a chiral centre.
Complete Step-by-Step solution:
Chirality is defined as a 'chiral or stereo centre' object that is asymmetric and cannot be superimposed over its mirror image. This characteristic is known as chirality. This behaviour of chirality is also observed in several organic compounds. Chirality is due to molecules' three-dimensional or spatial arrangements.
If it is superimposable in its mirror image, a molecule is achiral. Many achiral molecules do have a symmetry plane or a symmetry centre. Achiral molecules containing a stereo enter are referred to as meso.
Now, let us write the reaction of the treatment of $ {C_6}{H_{12}} $ with $ HCl $
$ {C_6}{H_{12}} + HCl \to {C_6}{H_{13}} - Cl $
$ {C_6}{H_{12}} $ on treatment with $ HCl $ produces an achiral carbon compound as shown in the figure below
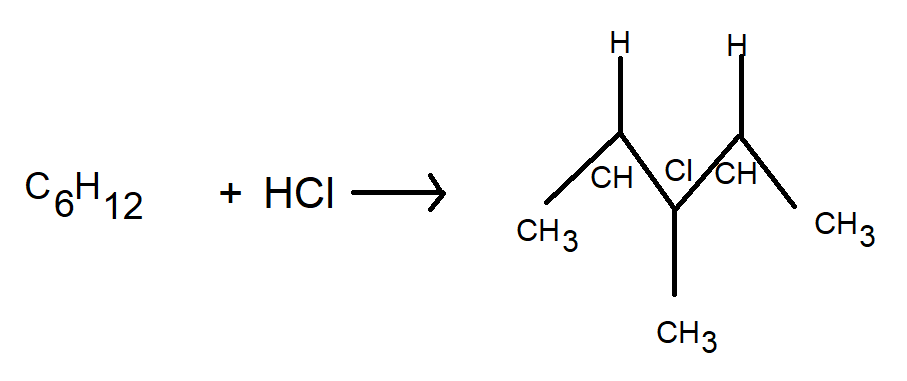
Here, $ {C_6}{H_{13}} - Cl $ is an optically inactive compound. This information was provided to us in the question. It is an achiral compound because we can observe in the figure that there are similar groups on both sides of the chlorine atom. Hence, the name achiral compound. It is said that a compound incapable of optical rotation is optically inactive. All pure achiral compounds are inactive optically.
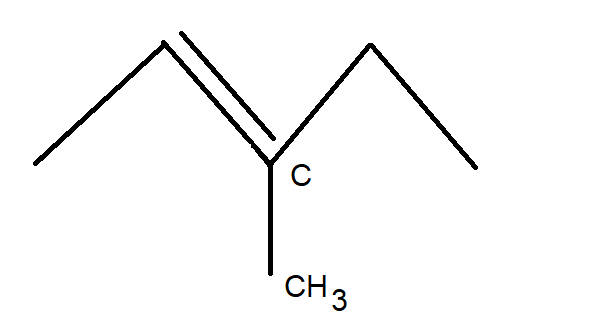
Now, using basic concepts of carbon compounds, let us try to draw the structure of the initial compound $ {C_6}{H_{12}} $ . The structure of $ {C_6}{H_{12}} $ can be drawn as shown in the figure below
Note:
If the light moves to the right, the optical activity of the dextrorotatory type is shown, and if it moves to the left, the optical activity of the laevorotatory type is shown. And as the band of light passes through Nicol's lens, it is unaltered. It's claimed to be inert optically. Optical activity, a substance's ability to rotate the polarization plane of a beam of light that passes through it. Since the specific rotation depends on the light's temperature and wavelength, it is also necessary to specify these quantities.
Complete Step-by-Step solution:
Chirality is defined as a 'chiral or stereo centre' object that is asymmetric and cannot be superimposed over its mirror image. This characteristic is known as chirality. This behaviour of chirality is also observed in several organic compounds. Chirality is due to molecules' three-dimensional or spatial arrangements.
If it is superimposable in its mirror image, a molecule is achiral. Many achiral molecules do have a symmetry plane or a symmetry centre. Achiral molecules containing a stereo enter are referred to as meso.
Now, let us write the reaction of the treatment of $ {C_6}{H_{12}} $ with $ HCl $
$ {C_6}{H_{12}} + HCl \to {C_6}{H_{13}} - Cl $
$ {C_6}{H_{12}} $ on treatment with $ HCl $ produces an achiral carbon compound as shown in the figure below

Here, $ {C_6}{H_{13}} - Cl $ is an optically inactive compound. This information was provided to us in the question. It is an achiral compound because we can observe in the figure that there are similar groups on both sides of the chlorine atom. Hence, the name achiral compound. It is said that a compound incapable of optical rotation is optically inactive. All pure achiral compounds are inactive optically.
Now, using basic concepts of carbon compounds, let us try to draw the structure of the initial compound $ {C_6}{H_{12}} $ . The structure of $ {C_6}{H_{12}} $ can be drawn as shown in the figure below

Note:
If the light moves to the right, the optical activity of the dextrorotatory type is shown, and if it moves to the left, the optical activity of the laevorotatory type is shown. And as the band of light passes through Nicol's lens, it is unaltered. It's claimed to be inert optically. Optical activity, a substance's ability to rotate the polarization plane of a beam of light that passes through it. Since the specific rotation depends on the light's temperature and wavelength, it is also necessary to specify these quantities.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




