
By Wurtz reaction which of the following will give you n- hexane?
A.1-chloro propane
B.2-chloro propane
C.Both of these
D.None of these
Answer
511.2k+ views
Hint: In Wurtz reaction, there is a formation of hydrocarbons by coupling reaction of alkyl halide in the presence of sodium metal. The sodium atom is reacted with the two alkyl halides with dry ether and a higher alkane should be obtained with sodium and halogen atoms. For example, the methyl bromide is reacting with sodium metal in the presence of ether there is a formation of higher alkane, which is ethane.
Complete answer:
The n- hexane can be prepared by using 1-chloro propane. Let’s see the general reaction of Wurtz reaction,
\[2RX + Na\xrightarrow{{Dry\,ether}}R - R + 2NaX\]
Similarly, 1-chloro propane is reacted in the presence of sodium metal and anhydrous dry ether, there is a formation of higher alkane, which is n-hexane. And the reaction can be written as,
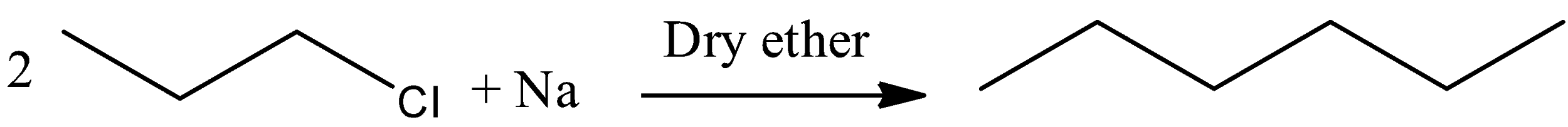
Hence, option (A) is correct.
When 2-chloro propane is reacted with sodium metal and dry ether, there is a formation of 2,3-dimethyl butane. Hence, the option (B) is incorrect.
Only, 1-chloro propane gives n-hexane. 2-chloro propane gives 2,3-dimethyl butane by Wurtz reaction. Hence, option (C) is incorrect.
Among the options, 1-chloro propane gives n-hexane. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We must have to know that the Wurtz reaction is used for the preparation of higher hydrocarbons by using two same or different alkyl halides with sodium metal and anhydrous ether. During the reaction, two alkyl groups are joined together, forming an alkane with sodium halide which is represented as NaX, and X is any halogen. But the Wurtz reaction is not used for the preparation of odd numbers of carbon. Because, there will be a formation of more than one product.
Complete answer:
The n- hexane can be prepared by using 1-chloro propane. Let’s see the general reaction of Wurtz reaction,
\[2RX + Na\xrightarrow{{Dry\,ether}}R - R + 2NaX\]
Similarly, 1-chloro propane is reacted in the presence of sodium metal and anhydrous dry ether, there is a formation of higher alkane, which is n-hexane. And the reaction can be written as,
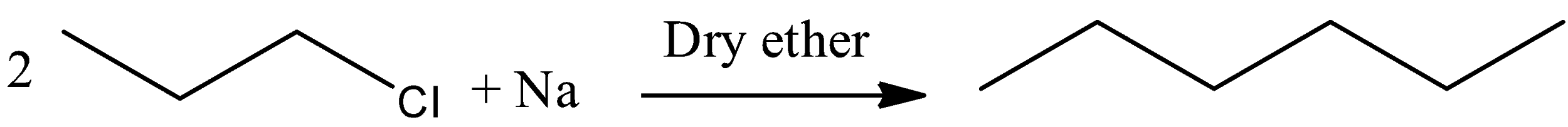
Hence, option (A) is correct.
When 2-chloro propane is reacted with sodium metal and dry ether, there is a formation of 2,3-dimethyl butane. Hence, the option (B) is incorrect.
Only, 1-chloro propane gives n-hexane. 2-chloro propane gives 2,3-dimethyl butane by Wurtz reaction. Hence, option (C) is incorrect.
Among the options, 1-chloro propane gives n-hexane. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We must have to know that the Wurtz reaction is used for the preparation of higher hydrocarbons by using two same or different alkyl halides with sodium metal and anhydrous ether. During the reaction, two alkyl groups are joined together, forming an alkane with sodium halide which is represented as NaX, and X is any halogen. But the Wurtz reaction is not used for the preparation of odd numbers of carbon. Because, there will be a formation of more than one product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




