
By the electrolysis of aqueous sodium succinate, ethene is formed at ………..along with ………..
A. Anode, ${ H }_{ 2 }$
B. Cathode, ${ H }_{ 2 }$
C. Anode, ${ CO }_{ 2 }$
D. Cathode, ${ CO }_{ 2 }$
Answer
597.3k+ views
Hint: Electrolysis: It is the process of separating a compound into its constituent particles by passing electricity through it when in the molten or aqueous state is known as electrolysis.
Complete step-by-step answer:
Kolbe’s electrolysis or Kolbe reaction is an organic reaction named after Adolph Wilhelm Hermann Kolbe. The Kolbe reaction is basically a decarboxylative dimerization and proceeds by a radical reaction mechanism.
In this reaction, an aqueous solution of sodium or potassium salt of a carboxylic acid is electrolyzed wherein dissociation of the salt into carboxylate ion and sodium or potassium ions take place.
At cathode, water gets converted to hydroxide ions and hydrogen gas.
${ 2H }_{ 2 }{ O }{ +2e^{ - } }{ \rightarrow 2OH^{ - } }{ +H }_{ 2 }$
At anode, the acetate ion donates an electron and gets converted to acetate radical. This acetate radical further releases carbon dioxide gas.
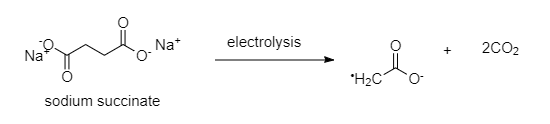
The complete reaction will take place as follows:
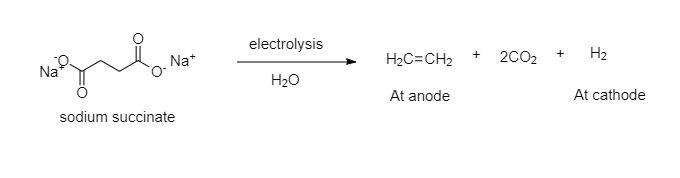
Hence, by the electrolysis of aqueous sodium succinate, ethene is formed at anode along with $H_2$.
The correct option is A.
Additional Information:
Methane cannot be prepared by this method as the alkane is formed by the combination of two alkyl free radicals. Therefore, the alkane should have a minimum of two carbon atoms.
Factors affecting electrolysis are:
1) Effect of concentration
2) Nature of electrolyte
3) Nature of electrodes
Note: The possibility to make a mistake is that you can choose option B. As we know that oxidation occurs at the anode and reduction occurs at the cathode. So, the ethene formed will be at anode, not cathode.
Complete step-by-step answer:
Kolbe’s electrolysis or Kolbe reaction is an organic reaction named after Adolph Wilhelm Hermann Kolbe. The Kolbe reaction is basically a decarboxylative dimerization and proceeds by a radical reaction mechanism.
In this reaction, an aqueous solution of sodium or potassium salt of a carboxylic acid is electrolyzed wherein dissociation of the salt into carboxylate ion and sodium or potassium ions take place.
At cathode, water gets converted to hydroxide ions and hydrogen gas.
${ 2H }_{ 2 }{ O }{ +2e^{ - } }{ \rightarrow 2OH^{ - } }{ +H }_{ 2 }$
At anode, the acetate ion donates an electron and gets converted to acetate radical. This acetate radical further releases carbon dioxide gas.

The complete reaction will take place as follows:

Hence, by the electrolysis of aqueous sodium succinate, ethene is formed at anode along with $H_2$.
The correct option is A.
Additional Information:
Methane cannot be prepared by this method as the alkane is formed by the combination of two alkyl free radicals. Therefore, the alkane should have a minimum of two carbon atoms.
Factors affecting electrolysis are:
1) Effect of concentration
2) Nature of electrolyte
3) Nature of electrodes
Note: The possibility to make a mistake is that you can choose option B. As we know that oxidation occurs at the anode and reduction occurs at the cathode. So, the ethene formed will be at anode, not cathode.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




