
Buckminsterfullerene is an allotropic form of:
(A) phosphorus
(B) Sulphur
(C) carbon
(D) tin
Answer
578.4k+ views
Hint: Some substances exist in more than one physical form. They differ in the arrangement of atoms in crystalline solid. Such substances are called allotropes.
They differ in physical properties such as color, hardness but usually alike in most chemical properties.
Complete step by step answer:
Element carbon exists in three different allotropic forms.
They are Diamond, Graphite and Buckminsterfullerene.
Buckminsterfullerene is an allotropic form of carbon.
So, the correct answer is “Option C”.
Additional Information:
This isomer is also known as Buckminsterfullerene has a molecular formula as ${C_{60}}$ and has a shape of soccer ball i.e., hollow sphere.
There are sixty equidistant spaces on the sphere that are occupied by C-atoms.
Each c-atom of fullerene is $s{p^2}$ hybridized
Electronic configuration of carbon is, $s{p^2}2{s^2}2p_x^12p_y^12p_z^1$
C-atom at ground state
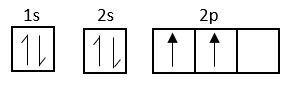
At excited state
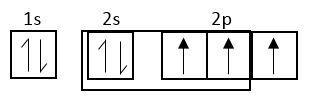
Hybridized state
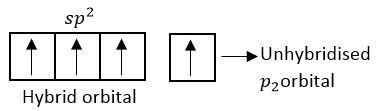
$2{p_z}$ orbitals form delocalized molecular orbital.
They spread over the complete structure of fullerene.
Carbon atoms are arranged in hexagons and pentagons and form hollow spheres.
Fullerene is observed in root.
When a high power laser was focused on carbon fullerene is formed.
Note:
Compounds of fullerene ${K_{35}}{C_{60}}$ act as superconductors of electricity.
It reacts with transition metal to form a catalyst Nanotubes are made from fullerene and graphite.
They are used in electric conductors, molecular sensors and semiconductors.
(1) Allotropes of phosphorus are white phosphorus, Red phosphorus, black phosphorus.
(2) Allotropes of Sulphur are Rhombic Sulphur, monoclinic Sulphur, $\alpha $-sulphur, $\beta $-sulphur plastic sulphur etc.
(3) Tin exists in two forms i.e., white or beta and gray or alpha tin.
They differ in physical properties such as color, hardness but usually alike in most chemical properties.
Complete step by step answer:
Element carbon exists in three different allotropic forms.
They are Diamond, Graphite and Buckminsterfullerene.
Buckminsterfullerene is an allotropic form of carbon.
So, the correct answer is “Option C”.
Additional Information:
This isomer is also known as Buckminsterfullerene has a molecular formula as ${C_{60}}$ and has a shape of soccer ball i.e., hollow sphere.
There are sixty equidistant spaces on the sphere that are occupied by C-atoms.
Each c-atom of fullerene is $s{p^2}$ hybridized
Electronic configuration of carbon is, $s{p^2}2{s^2}2p_x^12p_y^12p_z^1$
C-atom at ground state

At excited state

Hybridized state

$2{p_z}$ orbitals form delocalized molecular orbital.
They spread over the complete structure of fullerene.
Carbon atoms are arranged in hexagons and pentagons and form hollow spheres.
Fullerene is observed in root.
When a high power laser was focused on carbon fullerene is formed.
Note:
Compounds of fullerene ${K_{35}}{C_{60}}$ act as superconductors of electricity.
It reacts with transition metal to form a catalyst Nanotubes are made from fullerene and graphite.
They are used in electric conductors, molecular sensors and semiconductors.
(1) Allotropes of phosphorus are white phosphorus, Red phosphorus, black phosphorus.
(2) Allotropes of Sulphur are Rhombic Sulphur, monoclinic Sulphur, $\alpha $-sulphur, $\beta $-sulphur plastic sulphur etc.
(3) Tin exists in two forms i.e., white or beta and gray or alpha tin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




