
Bromobenzene on treatment with fuming ${H_2}S{O_4}$ gave a compound which on treatment with $C{l_2}/AlC{l_3}$ gives (A). (A) on distillation with dil. ${H_2}S{O_4}$ gives (B), (B) on treatment with an eq of Mg in dry ether gives (C). (C) on treatment with $C{H_3}CHO$ followed by hydrolysis gives (D). (D) on treatment with $NaOI$ gives (E). Identify the structures of (A) and (E).
Answer
513.3k+ views
Hint: We have to know that bromobenzene is a haloarene. In bromobenzene, the aromatic ring is bonded with the bromine group. We have to know that in haloarenes, the halogen atoms are bonded with carbon atoms of the alkyl group. We have to know that the carbon atom is $s{p^3}$ hybridized.
Complete answer:
We can draw the structure of bromobenzene as,
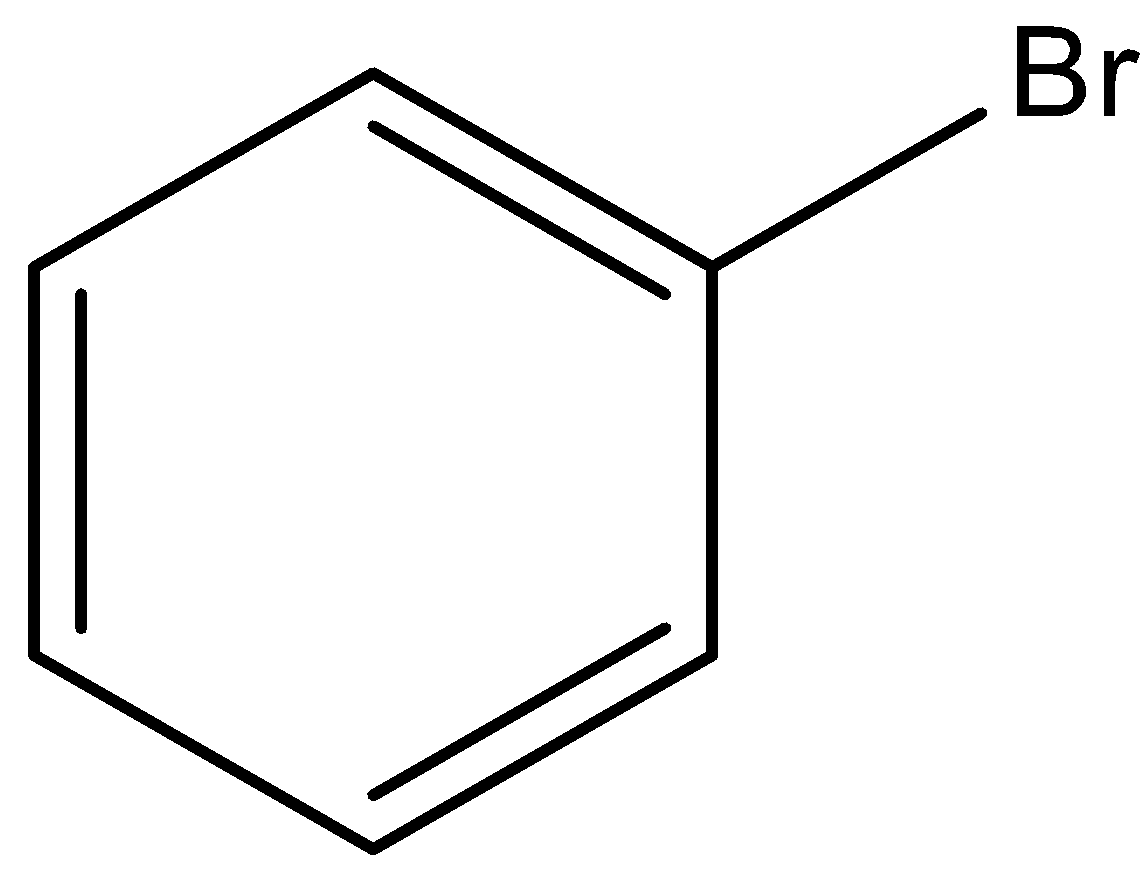
We have to know when bromobenzene undergoes sulfonation in the presence of fuming sulfuric acid to form 4-bromobenzenesulfonic acid. We can write the chemical reaction as,
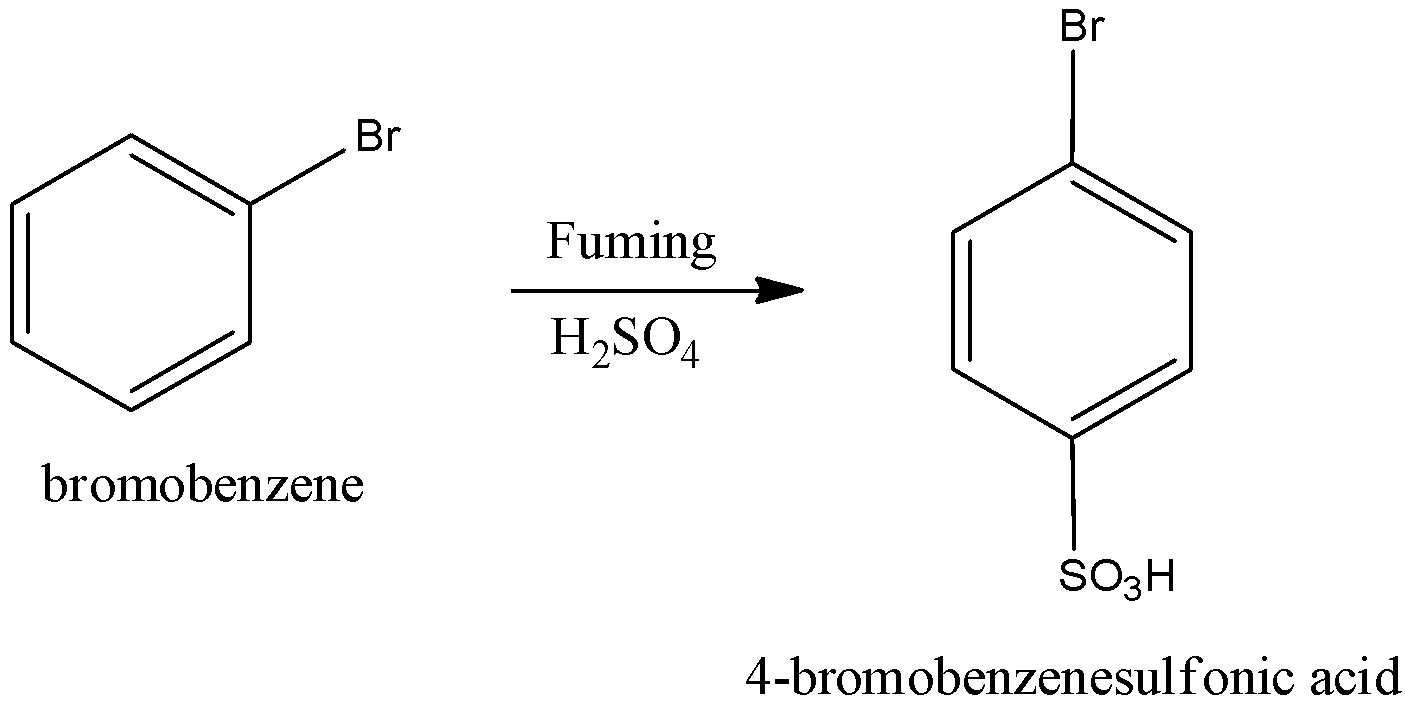
When 4-bromobenzenesulfonic acid undergoes reaction with $C{l_2}/AlC{l_3}$ we get the product 4-bromo-3-chlorobenzenesulfonic acid. We can write the chemical reaction as,
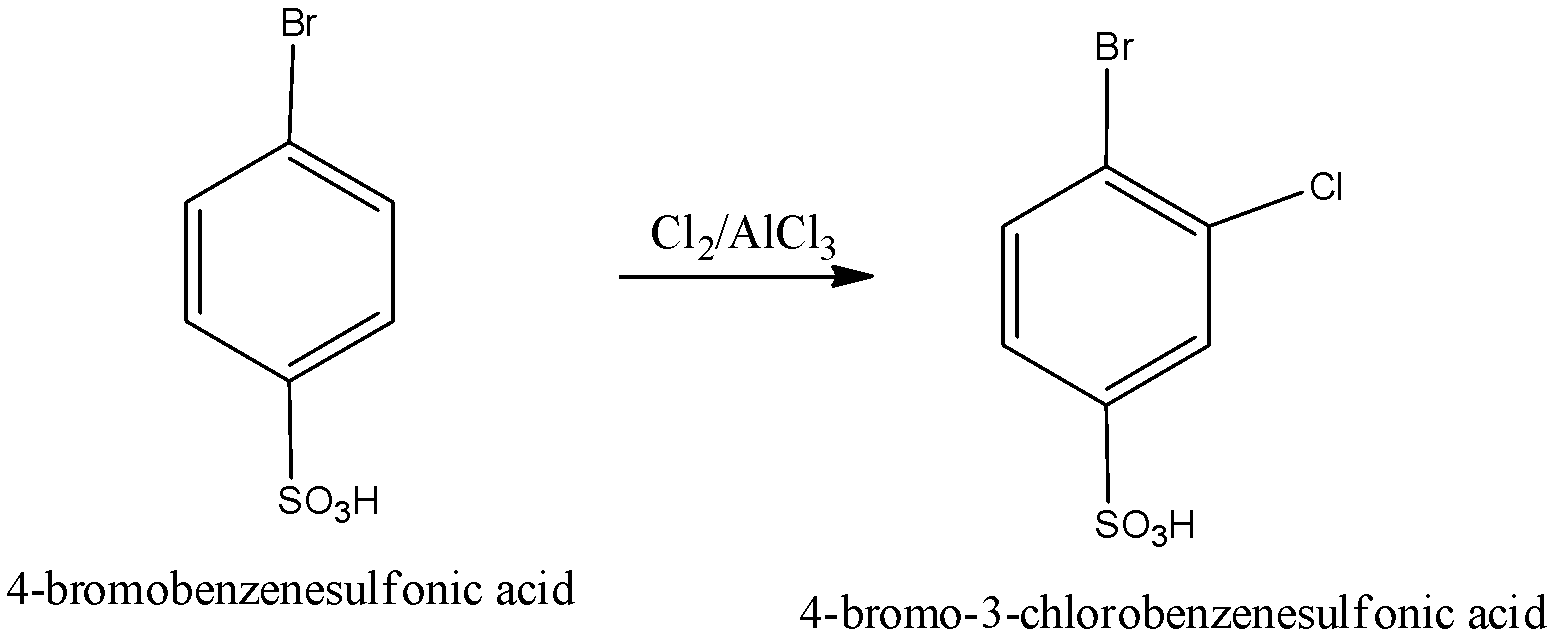
The compound (A) is identified as 4-bromo-3-chlorobenzenesulfonic acid.
When 4-bromo-3-chlorobenzenesulfonic acid undergoes reaction with dilute sulfuric acid, we get the product is 1-bromo-2-chlorobenzene. We can write the chemical reaction as,
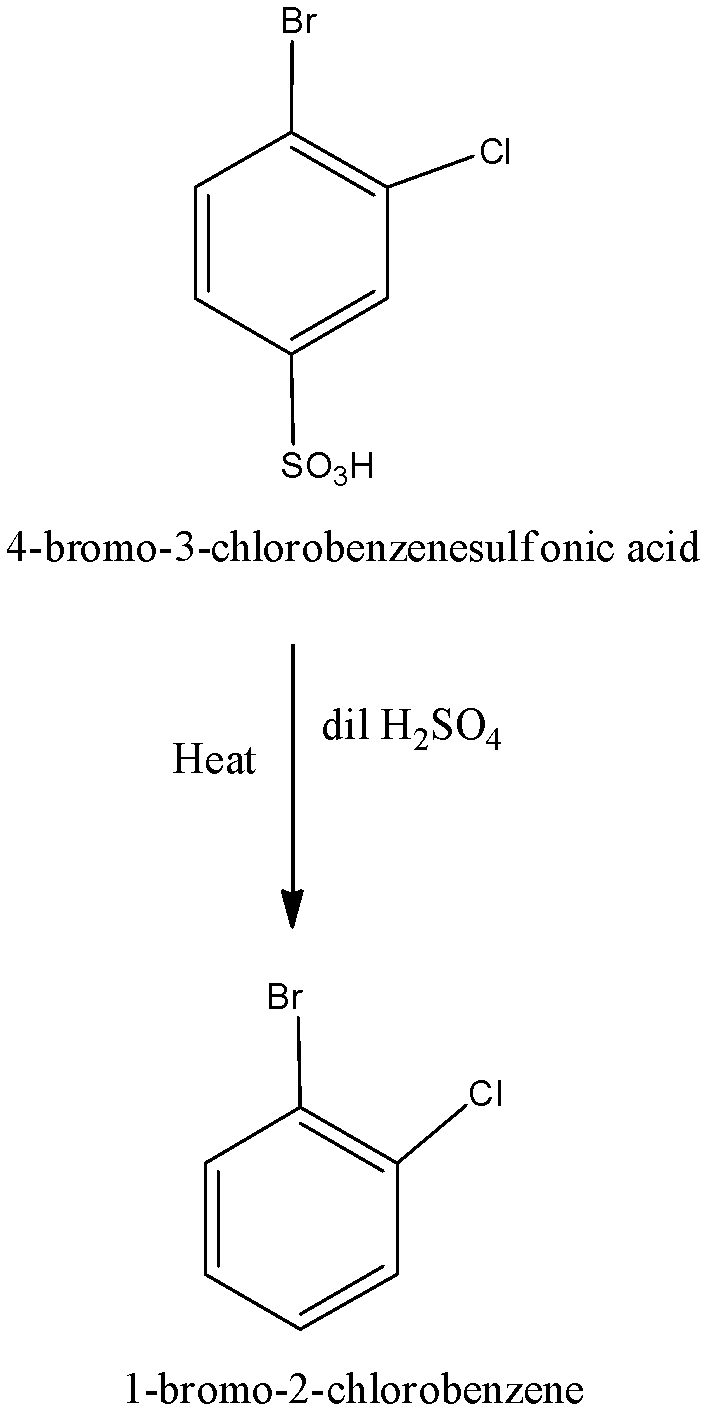
The compound (B) is identified as 1-bromo-2-chlorobenzene.
When 1-bromo-2-chlorobenzene undergoes reaction with magnesium in the presence of dry ether, we get the product (2-chlorophenyl) magnesium bromide. We can write the reaction as,
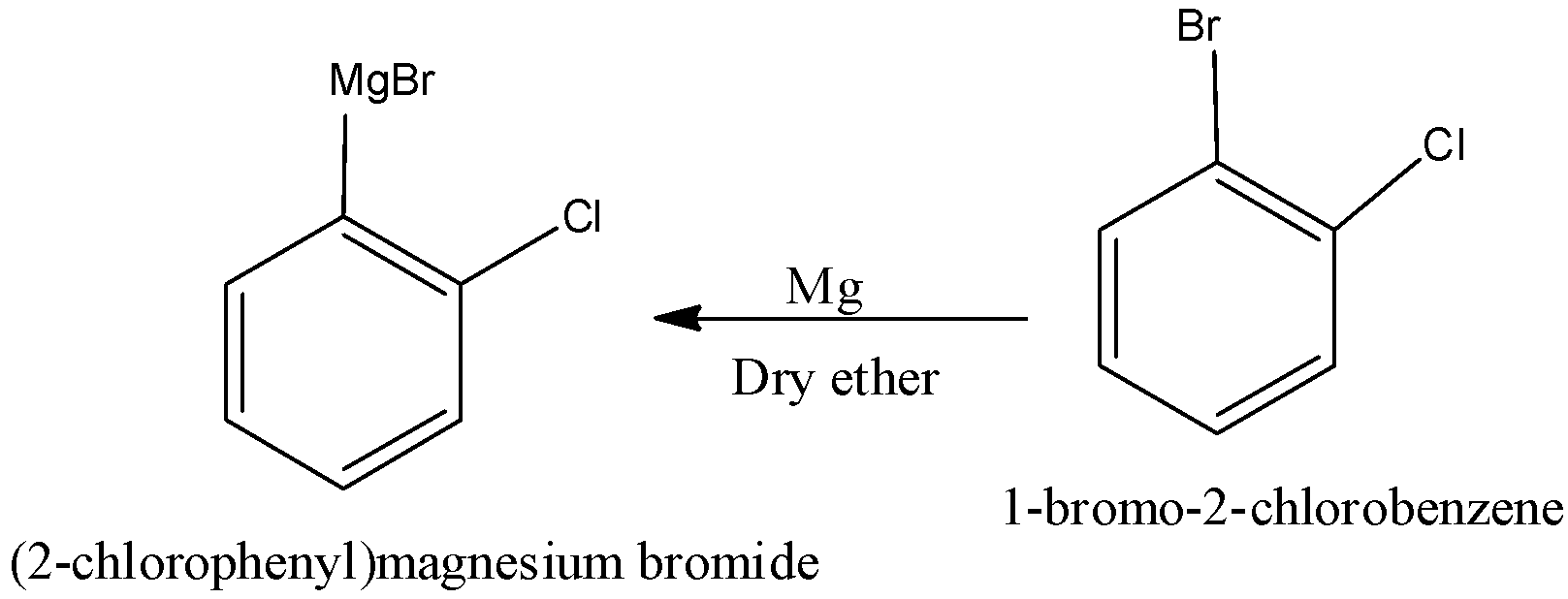
The compound (C) is identified as (2-chlorophenyl) magnesium bromide.
When (2-chlorophenyl) magnesium bromide undergoes reaction with acetaldehyde that is followed hydrolysis forms 1-(2-chlorophenyl) ethanol. We can write the reaction as,
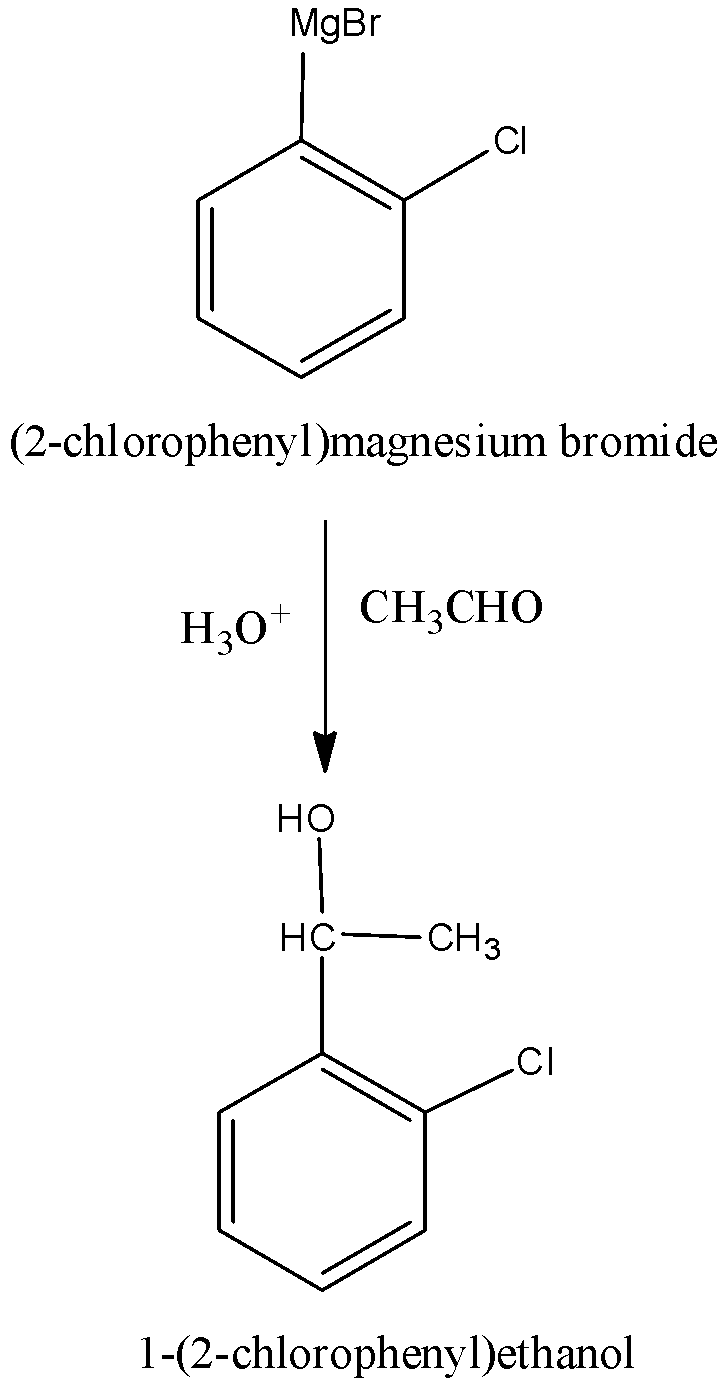
The compound (D) is identified as 1-(2-chlorophenyl) ethanol.
When 1-(2-chlorophenyl) ethanol undergoes oxidation reaction with NaOI, the product formed is a ketone. We can write the chemical reaction as,
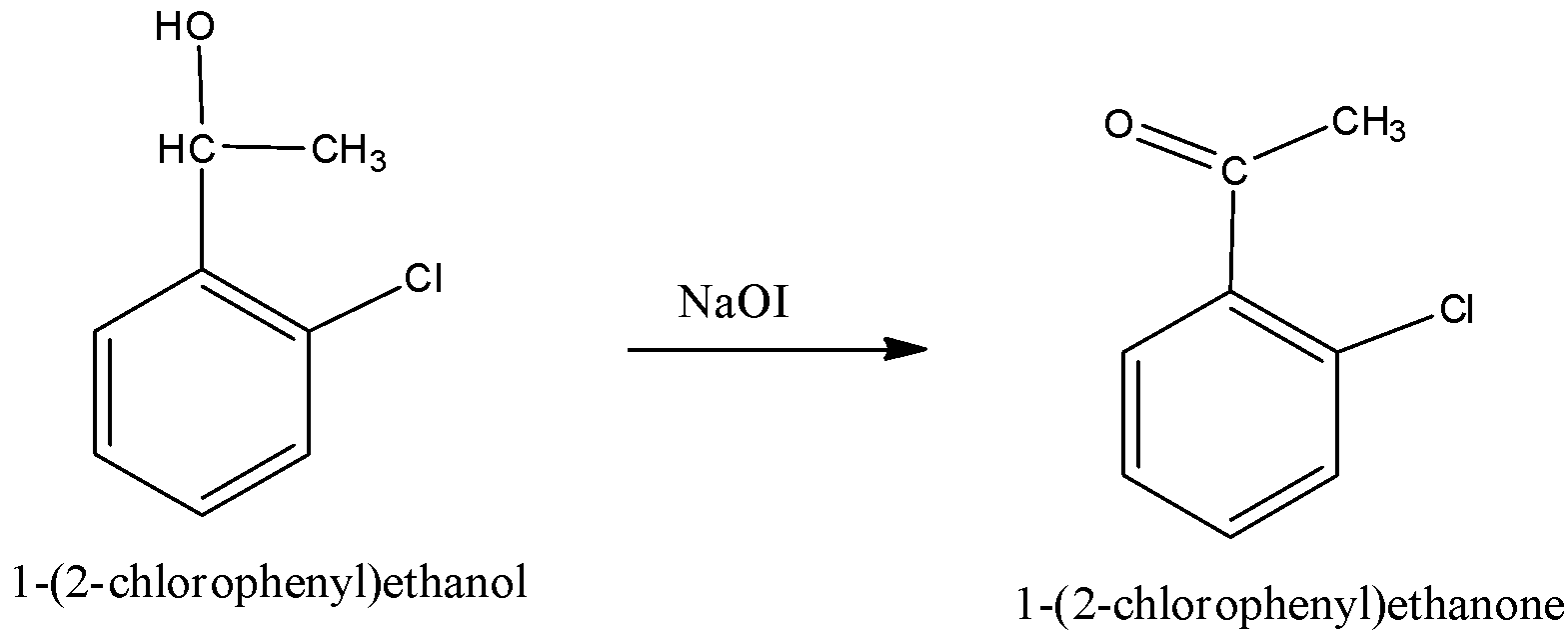
The compound (E) is identified as 1-(2-chlorophenyl) ethanone.
From the above chemical reactions, we have identified structure (A) as 4-bromo-3-chlorobenzenesulfonic acid and structure (E) as 1-(2-chlorophenyl) ethanone.
Note:
We can prepare bromobenzene by direct bromination of benzene. We have to know that bromobenzene is a closed chain hydrocarbon and it does not take part in $S{N_2}$substitution reactions. This compound is aromatic in nature and has a sweet odor. Bromobenzene undergoes mono haloarene.
Complete answer:
We can draw the structure of bromobenzene as,

We have to know when bromobenzene undergoes sulfonation in the presence of fuming sulfuric acid to form 4-bromobenzenesulfonic acid. We can write the chemical reaction as,

When 4-bromobenzenesulfonic acid undergoes reaction with $C{l_2}/AlC{l_3}$ we get the product 4-bromo-3-chlorobenzenesulfonic acid. We can write the chemical reaction as,

The compound (A) is identified as 4-bromo-3-chlorobenzenesulfonic acid.
When 4-bromo-3-chlorobenzenesulfonic acid undergoes reaction with dilute sulfuric acid, we get the product is 1-bromo-2-chlorobenzene. We can write the chemical reaction as,

The compound (B) is identified as 1-bromo-2-chlorobenzene.
When 1-bromo-2-chlorobenzene undergoes reaction with magnesium in the presence of dry ether, we get the product (2-chlorophenyl) magnesium bromide. We can write the reaction as,

The compound (C) is identified as (2-chlorophenyl) magnesium bromide.
When (2-chlorophenyl) magnesium bromide undergoes reaction with acetaldehyde that is followed hydrolysis forms 1-(2-chlorophenyl) ethanol. We can write the reaction as,

The compound (D) is identified as 1-(2-chlorophenyl) ethanol.
When 1-(2-chlorophenyl) ethanol undergoes oxidation reaction with NaOI, the product formed is a ketone. We can write the chemical reaction as,

The compound (E) is identified as 1-(2-chlorophenyl) ethanone.
From the above chemical reactions, we have identified structure (A) as 4-bromo-3-chlorobenzenesulfonic acid and structure (E) as 1-(2-chlorophenyl) ethanone.
Note:
We can prepare bromobenzene by direct bromination of benzene. We have to know that bromobenzene is a closed chain hydrocarbon and it does not take part in $S{N_2}$substitution reactions. This compound is aromatic in nature and has a sweet odor. Bromobenzene undergoes mono haloarene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




