
How many Br groups present in compound H?
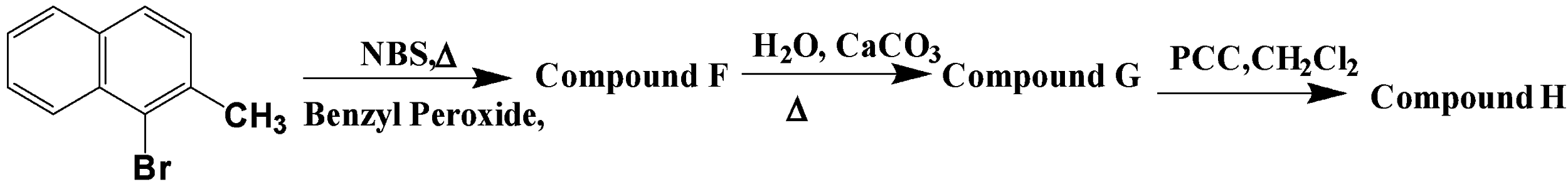
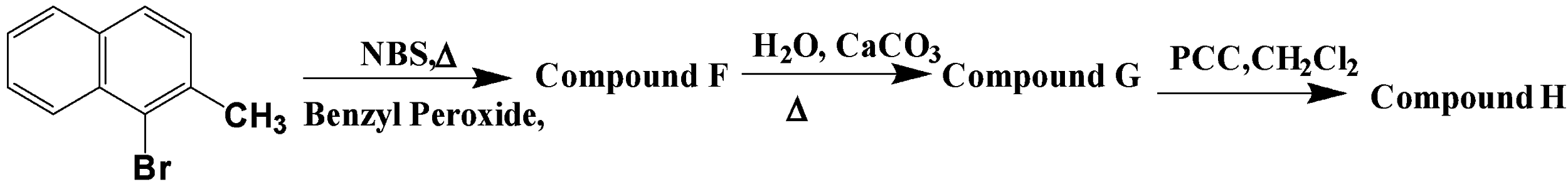
Answer
562.5k+ views
Hint: Organic chemistry is majorly formed by the chemical element and its compounds. Carbon being a tetravalent element can show versatility and can form compounds with many different properties. All these conversions from one compound to another can be carried with the help of specific reagents.
Complete step by step answer:
The question given has a chain of reactions going forward, each step uses a different reagent for the proceedings of the reactions. Each reagent has a different purpose and the compound changes form at each step as a result of the action of the reagents used.
In the first reaction, we see the use of NBS which is an oxidising agent and a ready source of the bromine atom. It is used as a source of bromine in radical reactions, it also uses benzoyl peroxide as a solvent for the compound. The second reagent used is calcium carbonate, it is used for the conversion of the excess bromine atom to the alcoholic group. This reaction takes place in the presence of water. After this reaction, we are left with compound G.
After the above series of reactions, the compound G is treated with PCC which is an oxidising agent and oxidises the alcohol group present in the compound to the ketone or aldehyde group.
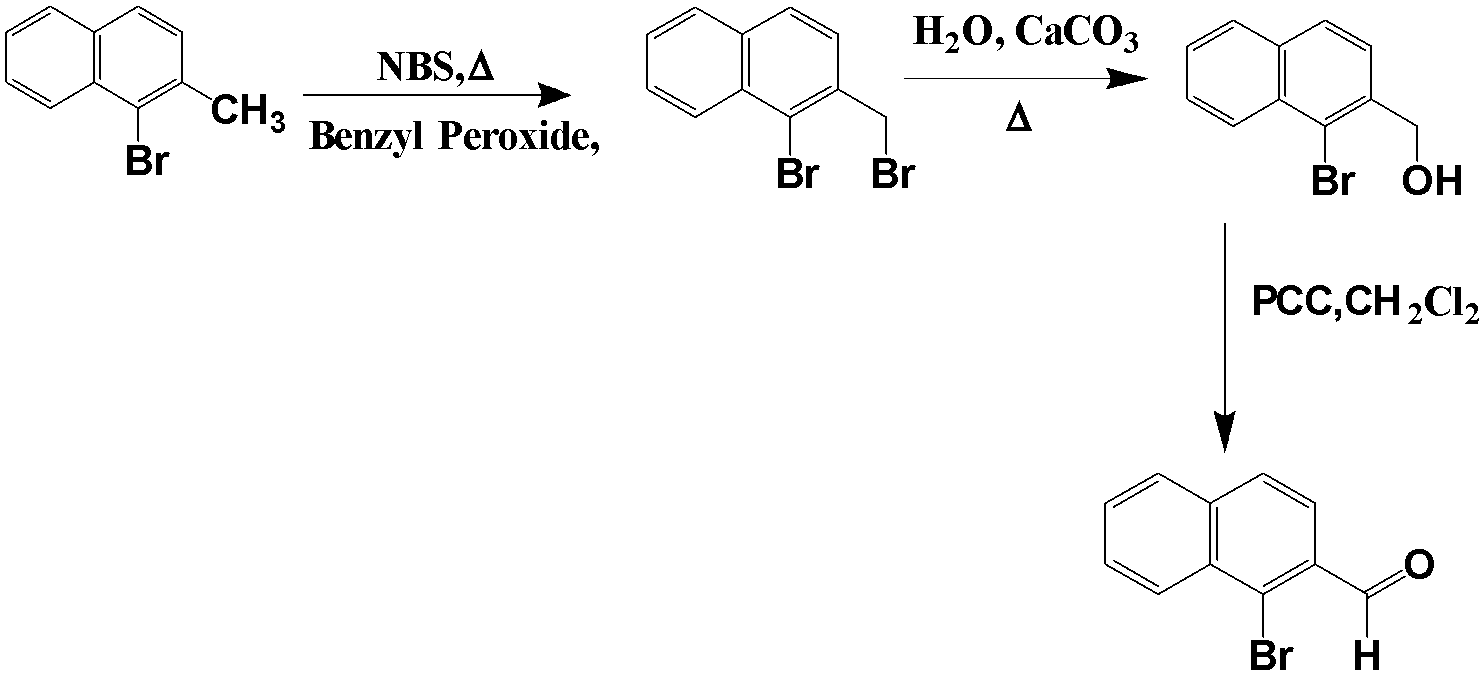
From the above figure, we can see that the compound H has only one bromine group attached to it.
Note: Benzene is an organic compound which has 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties.
The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. These are the relative positions for another main substituent already attached to the benzene.
Complete step by step answer:
The question given has a chain of reactions going forward, each step uses a different reagent for the proceedings of the reactions. Each reagent has a different purpose and the compound changes form at each step as a result of the action of the reagents used.
In the first reaction, we see the use of NBS which is an oxidising agent and a ready source of the bromine atom. It is used as a source of bromine in radical reactions, it also uses benzoyl peroxide as a solvent for the compound. The second reagent used is calcium carbonate, it is used for the conversion of the excess bromine atom to the alcoholic group. This reaction takes place in the presence of water. After this reaction, we are left with compound G.
After the above series of reactions, the compound G is treated with PCC which is an oxidising agent and oxidises the alcohol group present in the compound to the ketone or aldehyde group.

From the above figure, we can see that the compound H has only one bromine group attached to it.
Note: Benzene is an organic compound which has 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties.
The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. These are the relative positions for another main substituent already attached to the benzene.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




