
Both $C{O_2}$ and ${H_2}O$ contain polar covalent bonds but $C{O_2}$ is non-polar while ${H_2}O$ is polar because:
A. H atom is smaller than C atom
B. $C{O_2}$ is a linear molecule while ${H_2}O$ is an angular molecule
C. \[O - H\] bond is more polar than \[C - O\] bond
D. $C{O_2}$ contains multiple bonds while ${H_2}O$ has only single bonds
Answer
558.6k+ views
Hint:To solve this question, we must first understand some basic concepts about polarity and Covalent bonds. Then we need to assess the concept of Polar Covalent bond in such a way that we may conclude the correct reason behind the given statement and then only we can conclude the correct answer.
Complete step-by-step answer:Polar Covalent bond: Basically, a polar bond is a certain class of a covalent bond. A polar covalent bond is a bond that exists between two atoms consisting of electrons that are unevenly distributed. Due to this state, the molecules tend to have some electrical dipole moment wherein the two ends are either slightly positive or negative.
Electronegativity is the tendency of an atom to attract a shared pair of an electron towards itself. It has no units, it is a tendency. The covalent bond formed between two atoms in molecules whose electronegative difference exists is known as a polar covalent bond.
Step 1: The structure of are as follows:
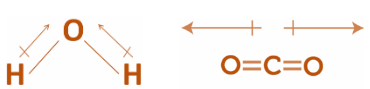
Step 2: By Going through the above explanation and considering the structure of both $C{O_2}$ and ${H_2}O$ , we may conclude that:
In the structure of $C{O_2}$ , the arrangement is a liner kind of linear arrangement in which polar bonds are arranged in such a way that the \[C - O\] bond polarities cancel each other.
Whereas, in the structure of ${H_2}O$ molecules, the arrangement is a linear kind of angular arrangement \[O - H\] bond polarities do not cancel one another.
Step 3: Hence we can conclude that the most correct reason will be:
$C{O_2}$ is a linear molecule while ${H_2}O$ is an angular molecule
And therefore, the correct answer is Option B.
Note:The polarity of a covalent bond can be explained by a physical quantity called Dipole moment \[(\mu )\] . The dipole moment is defined as the product of charge and distance of separation of charge.
Complete step-by-step answer:Polar Covalent bond: Basically, a polar bond is a certain class of a covalent bond. A polar covalent bond is a bond that exists between two atoms consisting of electrons that are unevenly distributed. Due to this state, the molecules tend to have some electrical dipole moment wherein the two ends are either slightly positive or negative.
Electronegativity is the tendency of an atom to attract a shared pair of an electron towards itself. It has no units, it is a tendency. The covalent bond formed between two atoms in molecules whose electronegative difference exists is known as a polar covalent bond.
Step 1: The structure of are as follows:

Step 2: By Going through the above explanation and considering the structure of both $C{O_2}$ and ${H_2}O$ , we may conclude that:
In the structure of $C{O_2}$ , the arrangement is a liner kind of linear arrangement in which polar bonds are arranged in such a way that the \[C - O\] bond polarities cancel each other.
Whereas, in the structure of ${H_2}O$ molecules, the arrangement is a linear kind of angular arrangement \[O - H\] bond polarities do not cancel one another.
Step 3: Hence we can conclude that the most correct reason will be:
$C{O_2}$ is a linear molecule while ${H_2}O$ is an angular molecule
And therefore, the correct answer is Option B.
Note:The polarity of a covalent bond can be explained by a physical quantity called Dipole moment \[(\mu )\] . The dipole moment is defined as the product of charge and distance of separation of charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




