
What is the bondline notation for cyclohexane?
Answer
524.1k+ views
Hint: Organic molecules are represented by a variety of notations. While a molecular formula only shows the forms and amounts of elements found in a molecule, an extended structural notation structure may be used to specify almost all of the compositional properties of an organic material.
Complete step by step answer:
We need to know that the bond-line notation, which uses "zig-zag" lines to describe the bonds that occur between consecutive carbon atoms, is used to create images by removing individual structural components from an extended, VSEPR, or condensed frame. As a result, relative to the systems from which they are derived, bond-line structures are much smaller in scale. Specifically, when constructing a bond-line structure from an illustration drawn using a more precise notation, all lone pairs, non-metal/hydrogen bonds, and all elemental symbols that signify carbon, C, and all hydrogen, H, elemental symbols that are connected with carbon atoms should be omitted. These remaining single bonds are expressed in a bond-line arrangement using "zig-zag" lines similar to those used in VSEPR notation. Since, as previously said, the second atom in any organic structure can be bound to either "side" of the first elemental symbol that is written, this "zig-zag" pattern can be started from either an upward or downward angle.
Hence, the bond-line notation of cyclohexane would is as follows:
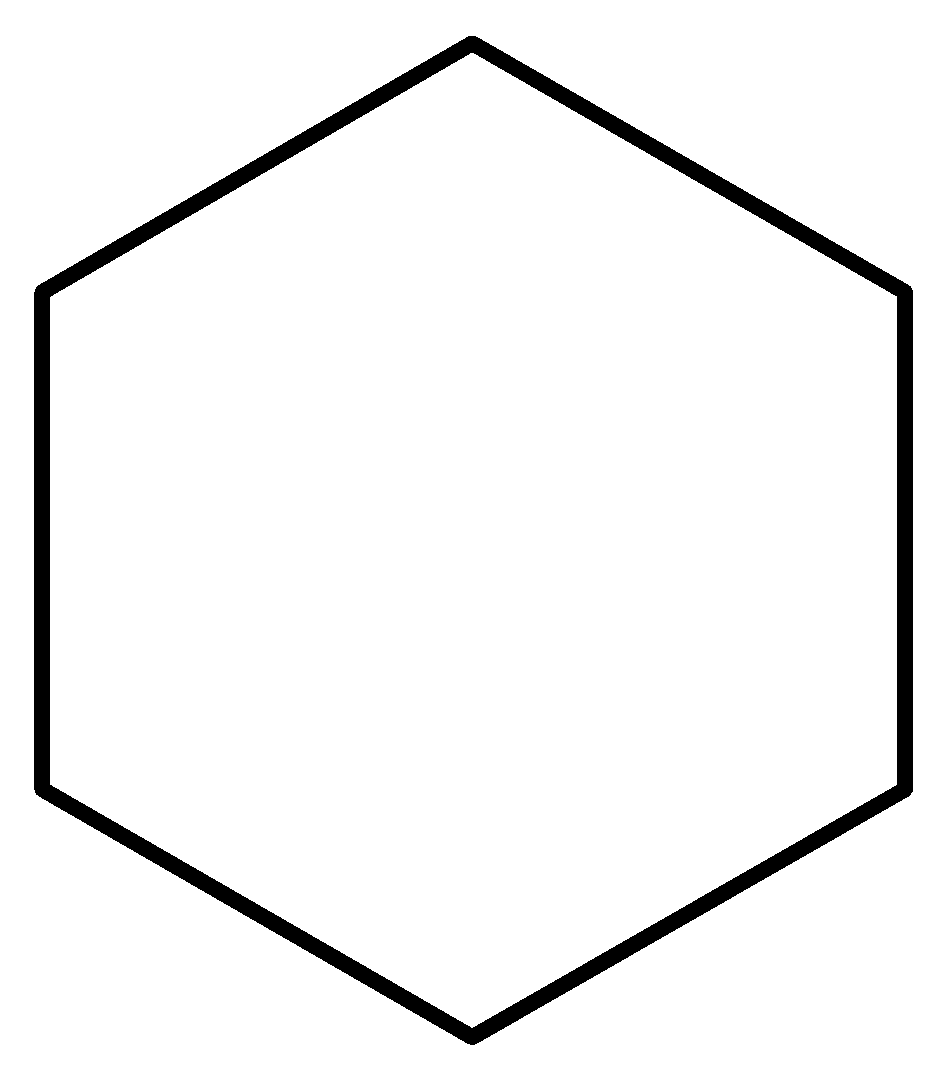
Fig: Bond-line notation of cyclohexane
additional information: The elemental symbols displayed in the accompanying structural representation signify the types of elements that are present. Counting the number of times each symbol is written in a molecular image can be used to determine the quantity of each element as well as the total number of bonds and lone pairs contained in a molecule.
A carbon atom is known to be found at every point where two or more bonds intersect, as well as at the end of every line that is not directly connected with a heteroatom, in bond-line notation. In addition, each carbon atom is thought to have enough hydrogen bonds to reach the preferred four-bond structure. An interpretation of normal non-metal bonding arrangements can be used to infer the total number of bonds and lone pairs present in a molecule, as well as the connectivity of each electron, bond, and lone pair. Finally, the number of lines drawn between any two atoms in the accompanying bond-line picture can be used to evaluate the forms of bonds present in a molecule.
Note: Although bond-line structures are more difficult to understand than extended, VSEPR, and simplified representations, bond-line structures can still be used to specify virtually all of a molecule's compositional characteristics.
Complete step by step answer:
We need to know that the bond-line notation, which uses "zig-zag" lines to describe the bonds that occur between consecutive carbon atoms, is used to create images by removing individual structural components from an extended, VSEPR, or condensed frame. As a result, relative to the systems from which they are derived, bond-line structures are much smaller in scale. Specifically, when constructing a bond-line structure from an illustration drawn using a more precise notation, all lone pairs, non-metal/hydrogen bonds, and all elemental symbols that signify carbon, C, and all hydrogen, H, elemental symbols that are connected with carbon atoms should be omitted. These remaining single bonds are expressed in a bond-line arrangement using "zig-zag" lines similar to those used in VSEPR notation. Since, as previously said, the second atom in any organic structure can be bound to either "side" of the first elemental symbol that is written, this "zig-zag" pattern can be started from either an upward or downward angle.
Hence, the bond-line notation of cyclohexane would is as follows:

Fig: Bond-line notation of cyclohexane
additional information: The elemental symbols displayed in the accompanying structural representation signify the types of elements that are present. Counting the number of times each symbol is written in a molecular image can be used to determine the quantity of each element as well as the total number of bonds and lone pairs contained in a molecule.
A carbon atom is known to be found at every point where two or more bonds intersect, as well as at the end of every line that is not directly connected with a heteroatom, in bond-line notation. In addition, each carbon atom is thought to have enough hydrogen bonds to reach the preferred four-bond structure. An interpretation of normal non-metal bonding arrangements can be used to infer the total number of bonds and lone pairs present in a molecule, as well as the connectivity of each electron, bond, and lone pair. Finally, the number of lines drawn between any two atoms in the accompanying bond-line picture can be used to evaluate the forms of bonds present in a molecule.
Note: Although bond-line structures are more difficult to understand than extended, VSEPR, and simplified representations, bond-line structures can still be used to specify virtually all of a molecule's compositional characteristics.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




