
What is the Bohr-Bury scheme of arrangement of electrons in an atom?
Answer
492.6k+ views
Hint: Electronic configuration of an atom is governed by a number of rules which includes: Aufbau principle, Bohr-Bury rule, Pauli exclusion principle and Hund’s rule. All these rules are employed to write the correct electronic configuration of any atom.
Complete answer:
Bohr-Bury rules are used to explain some important aspects of writing electronic configuration. According to this rule-
The energy of any orbital is determined with the help of numbers $n$ and $l$.
The filling of electrons in the orbital takes place according to the increasing order of $\left( {n + l} \right)$.
For example: energy of $3d$ orbital is $\left( {3 + 2 = 5} \right)$, while the energy of $4s$ orbital is $\left( {4 + 0 = 4} \right)$. Hence, according to Bohr-Bury rules energy of $3d$ is more than $4s$ orbital hence, electrons first fill in $3d$ orbital then fill into $4s$ orbital.
In case, if two orbitals possess the same energy level then the electron fills into an orbital which is associated with a lower value of $n$.
For example: the energy level of $2p$ orbital is $\left( {2 + 1 = 3} \right)$, and the energy level of $3s$ orbital is $\left( {3 + 1 = 3} \right)$. In this case the energy level of both the orbital is the same but electron first fills into $2p$ orbital because the value of $n$ is smaller in $2p$.
This rule helps in identifying the fact that an orbital with higher value of principal quantum number $n$, may have lower energy level than orbitals with lower value of principal quantum number.
Bohr-Bury states that the maximum capacity of a shell to hold electrons in them is equal to $2{n^2}$. Where $n$ describes the orbital energy of electrons.
Outermost shell of an atom must contain a maximum of $8$ electrons to attain stability.
Electron filling proceeds to another shell until its inner shell is completely filled.
Bohr-Bury scheme of arrangement of electrons in an atom is shown as:
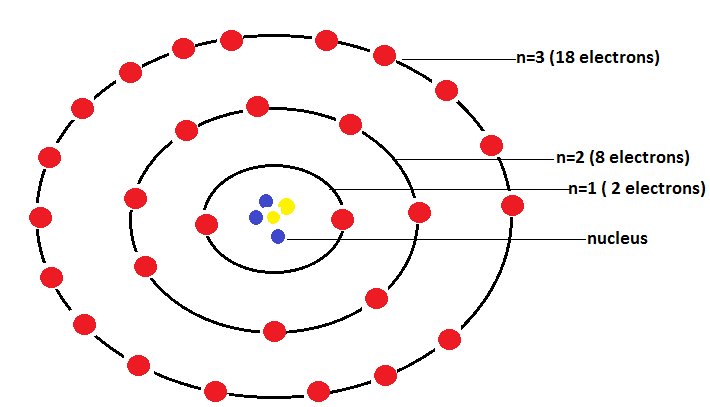
Note:
Aufbau helps in determining the energy of orbitals while Pauli explains the spin and maximum holding capacity of an orbital. Remember that a maximum of $2$ electrons must be present in one orbital while a maximum of $8$ present in orbit.
Complete answer:
Bohr-Bury rules are used to explain some important aspects of writing electronic configuration. According to this rule-
The energy of any orbital is determined with the help of numbers $n$ and $l$.
The filling of electrons in the orbital takes place according to the increasing order of $\left( {n + l} \right)$.
For example: energy of $3d$ orbital is $\left( {3 + 2 = 5} \right)$, while the energy of $4s$ orbital is $\left( {4 + 0 = 4} \right)$. Hence, according to Bohr-Bury rules energy of $3d$ is more than $4s$ orbital hence, electrons first fill in $3d$ orbital then fill into $4s$ orbital.
In case, if two orbitals possess the same energy level then the electron fills into an orbital which is associated with a lower value of $n$.
For example: the energy level of $2p$ orbital is $\left( {2 + 1 = 3} \right)$, and the energy level of $3s$ orbital is $\left( {3 + 1 = 3} \right)$. In this case the energy level of both the orbital is the same but electron first fills into $2p$ orbital because the value of $n$ is smaller in $2p$.
This rule helps in identifying the fact that an orbital with higher value of principal quantum number $n$, may have lower energy level than orbitals with lower value of principal quantum number.
Bohr-Bury states that the maximum capacity of a shell to hold electrons in them is equal to $2{n^2}$. Where $n$ describes the orbital energy of electrons.
Outermost shell of an atom must contain a maximum of $8$ electrons to attain stability.
Electron filling proceeds to another shell until its inner shell is completely filled.
Bohr-Bury scheme of arrangement of electrons in an atom is shown as:

Note:
Aufbau helps in determining the energy of orbitals while Pauli explains the spin and maximum holding capacity of an orbital. Remember that a maximum of $2$ electrons must be present in one orbital while a maximum of $8$ present in orbit.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




