
$B{F_3}$ dimerizes to form stable molecules . Enter 1 if true else enter 0 .
Answer
585.6k+ views
Hint: Dimerization is a process in which two molecules of similar chemical composition come together to form a single molecule also known as adduct . These units may be associated by covalent bonding or by non covalent forces .
Complete step by step answer: In case of $B{F_3}$ molecule boron has to satisfy its sixtet whereas fluorine has to satisfy its octet.
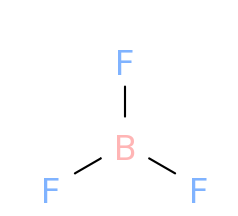
As we can see from the figure that when boron forms bonds with fluorine , boron has 6 electrons and fluorine has 8 electrons .
$B{F_3}$ does not form a dimer because boron is small in size and cannot accommodate more fluorine atoms therefore it cannot form a dimer .
Also in this molecule $p\Pi - p\Pi $ back bonding exists as boron has empty p - orbital (since it undergoes $s{p^2}$ hybridisation and has one vacant p - orbital ) and the p - orbital of fluorine contains lone pair of electrons , hence in this case boron acts as a lewis acid and fluorine acts as lewis base . Hence fluorine donates its lone pair of electrons to boron and this type of bonding is known as back bonding .
Therefore the answer is 0 , boron trifluoride does not dimerize .
Note: A molecule is said to be stable when all the elements present in the molecule have their octets satisfied ( in case of boron its sixtet has to be satisfied ) . Some molecules form dimers to become stable.
Complete step by step answer: In case of $B{F_3}$ molecule boron has to satisfy its sixtet whereas fluorine has to satisfy its octet.

As we can see from the figure that when boron forms bonds with fluorine , boron has 6 electrons and fluorine has 8 electrons .
$B{F_3}$ does not form a dimer because boron is small in size and cannot accommodate more fluorine atoms therefore it cannot form a dimer .
Also in this molecule $p\Pi - p\Pi $ back bonding exists as boron has empty p - orbital (since it undergoes $s{p^2}$ hybridisation and has one vacant p - orbital ) and the p - orbital of fluorine contains lone pair of electrons , hence in this case boron acts as a lewis acid and fluorine acts as lewis base . Hence fluorine donates its lone pair of electrons to boron and this type of bonding is known as back bonding .
Therefore the answer is 0 , boron trifluoride does not dimerize .
Note: A molecule is said to be stable when all the elements present in the molecule have their octets satisfied ( in case of boron its sixtet has to be satisfied ) . Some molecules form dimers to become stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




