
Betaine $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{11}}}{{\text{O}}_{\text{2}}}\text{N} $ occurs in beet sugar molasses. It is water soluble that melts with decomposition at $ \text{300}{{\text{ }}^{\text{0}}}\text{C} $ . It is unaffected by a base but reacts with hydrochloric acid to form a crystalline product $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{2}}}\text{N Cl} $ . It can be made from glycine with methyl iodide or treatment with chloroacetic acid with trimethyl amine. Draw the structure of betaine which will account for all the properties given above.
Answer
547.5k+ views
Hint: Betaine is a neutral chemical compound with positively charged cationic functional group such the quaternary ammonium or the phosphonium ions that bears no hydrogen atom and a negatively charged functional group such as the carboxylate group. It is a zwitterion.
Complete stepwise solution:
When Betaine $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{11}}}{{\text{O}}_{\text{2}}}\text{N} $ reacts with hydrochloric acid to form a crystalline product $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{2}}}\text{N Cl} $ , the reaction is shown as follows:
$ {{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{{\text{O}}_{\text{2}}}{\text{N}}\xrightarrow{{{\text{HCl}}}}{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}{\text{NCl}} $
The addition of hydrogen chloride to betiane to form the betiane hydrogen chloride salt indicates that betaine is a basic substance.
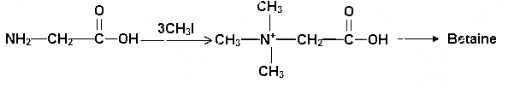
The above mechanism represents the mechanism for the formation of betiane from glycine. The glycine molecule is a small N-trimethylated amino acid. It is a zwitterion it cannot isomerize because there are no labile hydrogen atom attached to the nitrogen atom. This substance is called “glycine betiane” to distinguish it from the other betianes.
$ {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH + N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} \to {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} $
$ {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{NaI}} \to {\text{IC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{NaCl}} $
$ {\text{IC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} \to {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^{\text{ - }}} + {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{.HI}} $
The process of preparation of betaine from chloroacetic acid with triethylamine is an environmentally-friendly process to obtain high-purity betaine hydrochloride and the process consists of adding chloroacetic acid, a trimethylamine aqueous solution and Lewis base serving as a catalyst into a reaction vessel, reacting for 1 to 4 hours under the conditions that the temperature is 50 to 55 $ ^{\text{0}}\text{C} $ , and the pressure is $ \text{0}\text{.01 to 0}\text{.02 MPa} $ .
Note:
In many naturally occurring systems, the betaines serve as organic “osmolytes”. These are substances taken up for the environment for the protection against osmotic stress, draught, high salinity, and high temperature. Intracellular accumulation of betaine allows water retention in cells.
Complete stepwise solution:
When Betaine $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{11}}}{{\text{O}}_{\text{2}}}\text{N} $ reacts with hydrochloric acid to form a crystalline product $ {{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{2}}}\text{N Cl} $ , the reaction is shown as follows:
$ {{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{{\text{O}}_{\text{2}}}{\text{N}}\xrightarrow{{{\text{HCl}}}}{{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{2}}}{\text{NCl}} $
The addition of hydrogen chloride to betiane to form the betiane hydrogen chloride salt indicates that betaine is a basic substance.

The above mechanism represents the mechanism for the formation of betiane from glycine. The glycine molecule is a small N-trimethylated amino acid. It is a zwitterion it cannot isomerize because there are no labile hydrogen atom attached to the nitrogen atom. This substance is called “glycine betiane” to distinguish it from the other betianes.
$ {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH + N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} \to {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} $
$ {\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{NaI}} \to {\text{IC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{NaCl}} $
$ {\text{IC}}{{\text{H}}_{\text{2}}}{\text{COOH}}{\text{.N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} + {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}} \to {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^{\text{ - }}} + {\text{N}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{.HI}} $
The process of preparation of betaine from chloroacetic acid with triethylamine is an environmentally-friendly process to obtain high-purity betaine hydrochloride and the process consists of adding chloroacetic acid, a trimethylamine aqueous solution and Lewis base serving as a catalyst into a reaction vessel, reacting for 1 to 4 hours under the conditions that the temperature is 50 to 55 $ ^{\text{0}}\text{C} $ , and the pressure is $ \text{0}\text{.01 to 0}\text{.02 MPa} $ .
Note:
In many naturally occurring systems, the betaines serve as organic “osmolytes”. These are substances taken up for the environment for the protection against osmotic stress, draught, high salinity, and high temperature. Intracellular accumulation of betaine allows water retention in cells.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




