
What is the best way to convert 1-pentyne into Pentanal?
A) \[HgS{O_4}/{H_2}S{O_4}^ - \]
B) ${H_2}/Lindlar'scatalyst;$${O_3} - Zn - {H_2}O$
C) \[HI{O_4}/{H_2}O\]
D) \[B{H_3}:{H_2}{O_2}/NaOH\]
Answer
504.6k+ views
Hint: We need to know that the pentyne is an alkyne having triple bond is a type of hydrocarbon that is composed of carbon and hydrogen atoms having triple bond between carbon atoms while Pentanal is an aldehyde of five carbon atoms having an aldehydic functional group $ - CHO$.
Complete answer:
The conversion of 1-pentyne into Pentanal can be carried out in many ways but the best way to carry out this conversion is \[B{H_3}:{H_2}{O_2}/NaOH\]. The reaction begins with the concerted syn addition of B and $H$ across the double bond, with the boron adding to the less substituted carbon. In the second step, hydrogen peroxide and a base such as \[NaOH\] are added. The \[NaOH\] deprotonates the hydrogen peroxide which makes the conjugate base of hydrogen peroxide (a better nucleophile than \[{H_2}{O_2}\]itself). The resulting \[NaOH\] then attacks the boron. This sets up the key migration step, where the carbon-boron bond migrates to the oxygen bound to boron, breaking the weak oxygen-oxygen bond. The OH expelled then comes back to form a bond on the boron resulting in the deprotonated alcohol (alkoxide). The alkoxide is then protonated by water or some other comparably acidic species
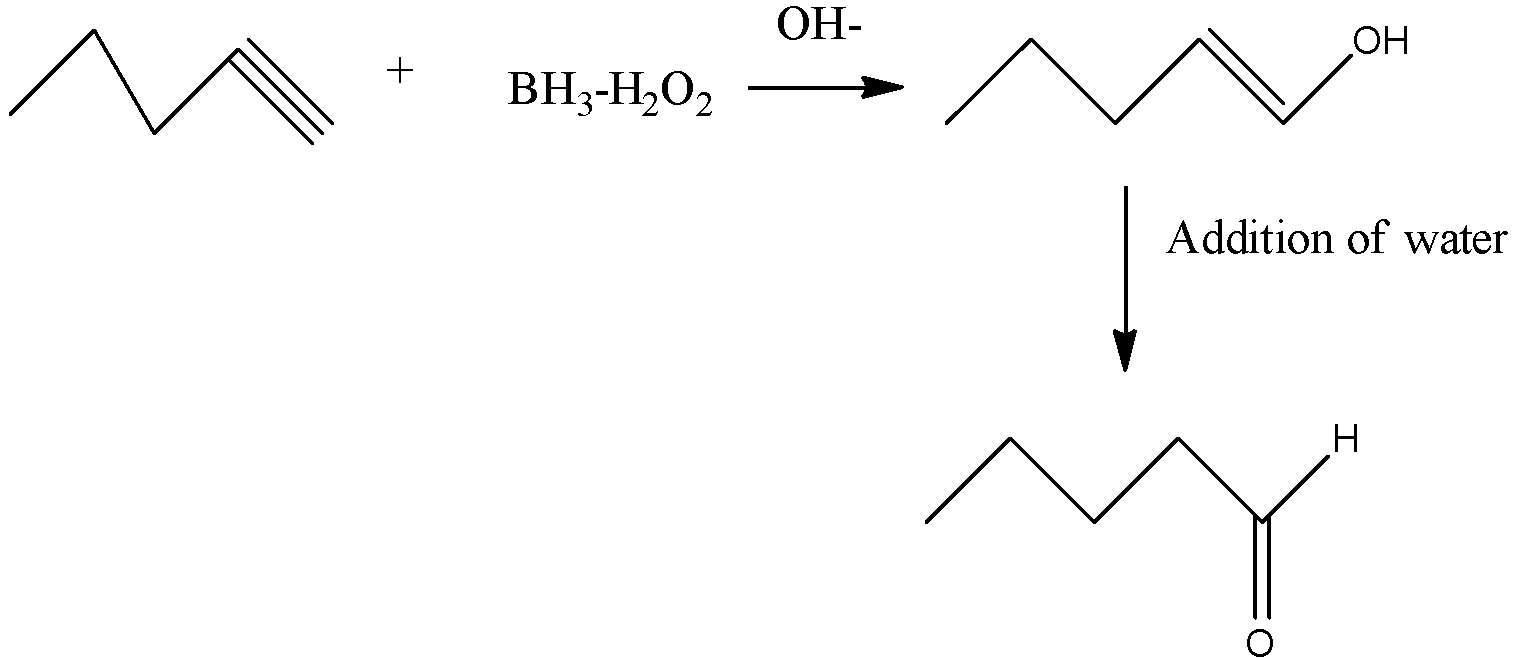
The boron byproduct will depend on the # of equivalents of \[B{H_3}\] used relative to the alkene. Here their molar ratio is $1:1$. One equivalent of \[B{H_3}\] can hydroborate up to $3$ equivalents of alkene.
So, the correct answer is “Option D”.
Note:
We need to know that the oxygen is always attached to the less substituted carbon (anti-Markovnikov). Furthermore the stereochemistry is always syn ($H$ and $OH$ add to the same side of the alkene). \[B{H_3} - THF\] is the same as \[B{H_3}\]. Tetrahydrofuran (THF) is merely a solvent. Sometimes \[{B_2}{H_6}\] is written, which is another form of \[B{H_3}\]. It behaves in exactly the same way as \[B{H_3}\].
Complete answer:
The conversion of 1-pentyne into Pentanal can be carried out in many ways but the best way to carry out this conversion is \[B{H_3}:{H_2}{O_2}/NaOH\]. The reaction begins with the concerted syn addition of B and $H$ across the double bond, with the boron adding to the less substituted carbon. In the second step, hydrogen peroxide and a base such as \[NaOH\] are added. The \[NaOH\] deprotonates the hydrogen peroxide which makes the conjugate base of hydrogen peroxide (a better nucleophile than \[{H_2}{O_2}\]itself). The resulting \[NaOH\] then attacks the boron. This sets up the key migration step, where the carbon-boron bond migrates to the oxygen bound to boron, breaking the weak oxygen-oxygen bond. The OH expelled then comes back to form a bond on the boron resulting in the deprotonated alcohol (alkoxide). The alkoxide is then protonated by water or some other comparably acidic species

The boron byproduct will depend on the # of equivalents of \[B{H_3}\] used relative to the alkene. Here their molar ratio is $1:1$. One equivalent of \[B{H_3}\] can hydroborate up to $3$ equivalents of alkene.
So, the correct answer is “Option D”.
Note:
We need to know that the oxygen is always attached to the less substituted carbon (anti-Markovnikov). Furthermore the stereochemistry is always syn ($H$ and $OH$ add to the same side of the alkene). \[B{H_3} - THF\] is the same as \[B{H_3}\]. Tetrahydrofuran (THF) is merely a solvent. Sometimes \[{B_2}{H_6}\] is written, which is another form of \[B{H_3}\]. It behaves in exactly the same way as \[B{H_3}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




