
Benzylamine is a stronger base than aniline because:
A) The lone pair of electrons on the nitrogen atom in benzylamine is delocalised.
B) The lone pair of electrons on the nitrogen atom in aniline is delocalised.
C) The lone pair of electrons on the nitrogen atom in aniline is not involved in resonance.
D) Benzylamine has a higher molecular mass than aniline.
Answer
575.7k+ views
Hint: A stronger base readily donates its pairs of electrons. Chemical formula of aniline and benzylamine is ${C_6}{H_5}N{H_2}$ and ${C_6}{H_5}C{H_2}N{H_2}$ respectively. In both lone pairs are present on nitrogen atoms. But in aniline, nitrogen is attached to the benzene ring whereas in benzylamine, nitrogen is attached to the saturated carbon.
Complete answer:
According to Lewis theory of acids and bases, a base is the one which has the availability of electrons to donate to an acid. Now, here we need to find the stronger base among benzylamine and aniline. A stronger base will be the one whose electrons are easily available for donation. Chemical formula of aniline is ${C_6}{H_5}N{H_2}$ and its structure is as given below:
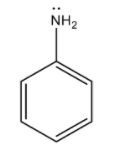
In this molecule, a lone pair of electrons is present on nitrogen atom (N) and this nitrogen atom is attached to a $s{p^2}$ hybridised carbon of the benzene ring. The lone pair of electrons present on nitrogen will involve in resonance with the benzene ring as:
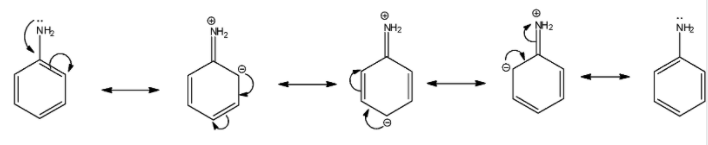
Thus, it is quite clear that due to resonance present in aniline, lone pair of electrons available are delocalised and hence will be less available for donation. This makes aniline a weaker base. Now, chemical formula of benzylamine is ${C_6}{H_5}C{H_2}N{H_2}$ and its structure is as given below:
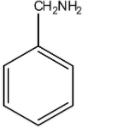
In this molecule, a lone pair of electrons is again present on nitrogen (N). But here, nitrogen is attached to a $s{p^3}$ hybridised carbon and not to the benzene ring. Hence, lone pairs of electrons on nitrogen are not involved in any resonance with the benzene ring. Therefore, lone pairs of electrons are localised here on nitrogen atoms and will be readily available for donation. Thus, benzylamine is a stronger base. Hence, benzylamine is a stronger base than aniline because the lone pair of electrons on the nitrogen atom in aniline is delocalised.
Thus, option B is correct.
Note: Both benzylamine and aniline are bases but relatively differs in their basicity. You must know that lower is the $p{K_b}$ value (i.e., numeric measurement of the basicity) , stronger is the base. The $p{K_b}$ value of benzylamine is 4.70 while that of aniline is 9.13.
Complete answer:
According to Lewis theory of acids and bases, a base is the one which has the availability of electrons to donate to an acid. Now, here we need to find the stronger base among benzylamine and aniline. A stronger base will be the one whose electrons are easily available for donation. Chemical formula of aniline is ${C_6}{H_5}N{H_2}$ and its structure is as given below:

In this molecule, a lone pair of electrons is present on nitrogen atom (N) and this nitrogen atom is attached to a $s{p^2}$ hybridised carbon of the benzene ring. The lone pair of electrons present on nitrogen will involve in resonance with the benzene ring as:

Thus, it is quite clear that due to resonance present in aniline, lone pair of electrons available are delocalised and hence will be less available for donation. This makes aniline a weaker base. Now, chemical formula of benzylamine is ${C_6}{H_5}C{H_2}N{H_2}$ and its structure is as given below:

In this molecule, a lone pair of electrons is again present on nitrogen (N). But here, nitrogen is attached to a $s{p^3}$ hybridised carbon and not to the benzene ring. Hence, lone pairs of electrons on nitrogen are not involved in any resonance with the benzene ring. Therefore, lone pairs of electrons are localised here on nitrogen atoms and will be readily available for donation. Thus, benzylamine is a stronger base. Hence, benzylamine is a stronger base than aniline because the lone pair of electrons on the nitrogen atom in aniline is delocalised.
Thus, option B is correct.
Note: Both benzylamine and aniline are bases but relatively differs in their basicity. You must know that lower is the $p{K_b}$ value (i.e., numeric measurement of the basicity) , stronger is the base. The $p{K_b}$ value of benzylamine is 4.70 while that of aniline is 9.13.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




