
Benzyl chloride is converted to _________ using sodium hydroxide solution.
a) Phenol
b) o-chloromethyl phenol
c) p-chloromethyl phenol
d) benzyl alcohol
Answer
605.4k+ views
Hint: For identifying the end product of a reaction, look at the reagent provided in the question. Sodium hydroxide solution or aqueous NaOH is a strong base. Therefore, this reaction will result in a substitution product.
Complete step by step answer:
The data provided to us in the question is –
Benzyl chloride – reactant. It is a benzyl halide, in which the halogen acts as a leaving group.
Nucleophile – Sodium hydroxide solution or aqueous NaOH. It is a very good base and a strong nucleophile
In organic chemistry, we can predict the product for a chemical reaction by looking at the reaction compound and the reagent. Since we have a benzylic halide with a strong nucleophile, the reaction will proceed via SN2 mechanism and a substitution product will be formed.
Therefore, the hydroxyl group replaces the chloride group.
The reaction can be represented as –
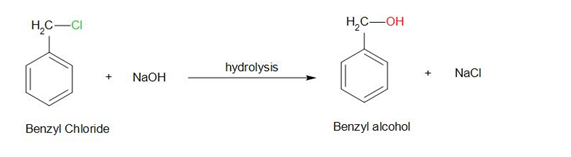
Therefore, the answer is – option (d) – Benzyl Alcohol.
Additional Information:
Benzylic halides are the compounds in which the halogen is bonded to a \[s{{p}^{3}}\] hybridized carbon atom next to an aromatic ring.
Note: There are two types of substitution reactions –
1) Substitution nucleophilic unimolecular
-It is also known as SN1 type reaction, because it is a first order reaction.
-It takes place in a polar protic solution.
-A weak nucleophile is needed in such reactions.
2) Substitution nucleophilic bimolecular
-It is also known as SN2 type reaction, because it is a second order reaction.
-It takes place in a polar aprotic solution.
-A strong nucleophile is needed in such reactions.
Complete step by step answer:
The data provided to us in the question is –
Benzyl chloride – reactant. It is a benzyl halide, in which the halogen acts as a leaving group.
Nucleophile – Sodium hydroxide solution or aqueous NaOH. It is a very good base and a strong nucleophile
In organic chemistry, we can predict the product for a chemical reaction by looking at the reaction compound and the reagent. Since we have a benzylic halide with a strong nucleophile, the reaction will proceed via SN2 mechanism and a substitution product will be formed.
Therefore, the hydroxyl group replaces the chloride group.
The reaction can be represented as –

Therefore, the answer is – option (d) – Benzyl Alcohol.
Additional Information:
Benzylic halides are the compounds in which the halogen is bonded to a \[s{{p}^{3}}\] hybridized carbon atom next to an aromatic ring.
Note: There are two types of substitution reactions –
1) Substitution nucleophilic unimolecular
-It is also known as SN1 type reaction, because it is a first order reaction.
-It takes place in a polar protic solution.
-A weak nucleophile is needed in such reactions.
2) Substitution nucleophilic bimolecular
-It is also known as SN2 type reaction, because it is a second order reaction.
-It takes place in a polar aprotic solution.
-A strong nucleophile is needed in such reactions.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




