
How is Benzene converted to Salicylic acid?
Answer
504.3k+ views
Hint: Salicylic acid is $2{\text{ - Hydroxybenzoic acid}}$. Therefore it contains a $OH$group and a $COOH$group. We have to substitute these two groups on the given benzene. For this we will convert benzene into phenol and will add carboxylic acid .
Complete answer:
Benzene can be converted into Salicylic acid using the following steps :
Step $1.$We will first convert Benzene to phenol. There are many through which it can be converted into phenol. One of them is mentioned below:
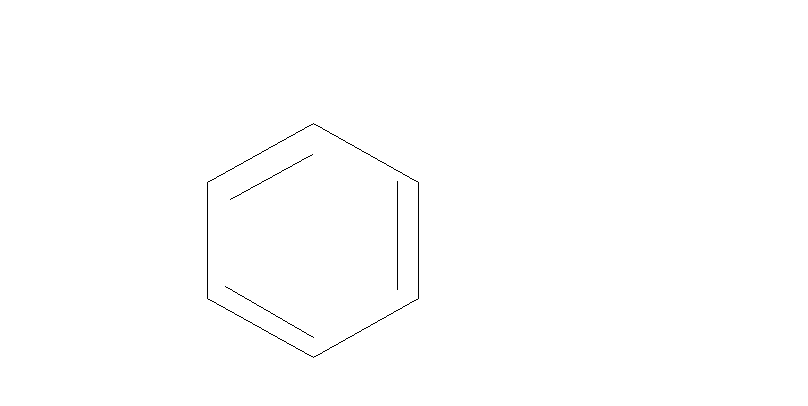 $\xrightarrow{{{H_2}S{O_4}}}$
$\xrightarrow{{{H_2}S{O_4}}}$
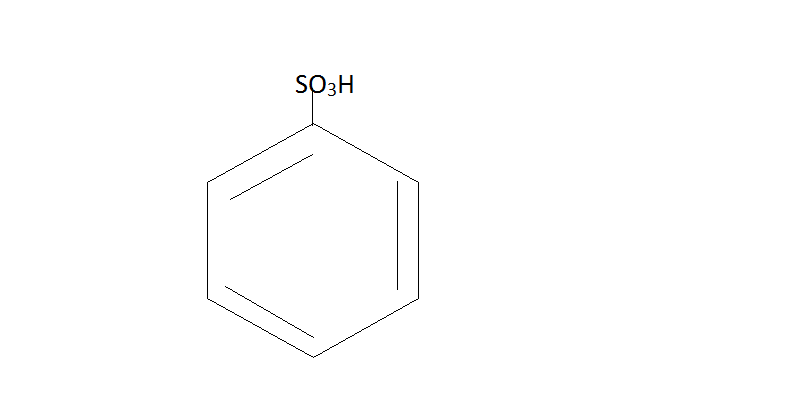 $\xrightarrow{{NaOH}}$
$\xrightarrow{{NaOH}}$
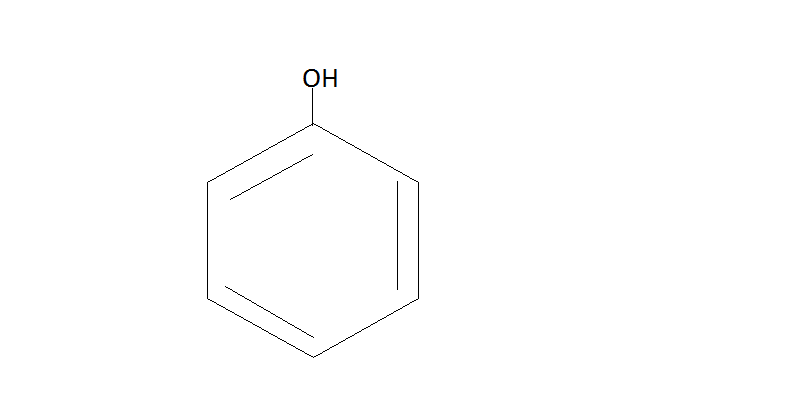
Benzene Benzene Sulfonic acid Phenol
Here Benzene reacts with sulfuric acid to form Benzene Sulfonic acid. Then this acid further reacts with $NaOH$at ${200^ \circ }C$ . This will give us phenol.
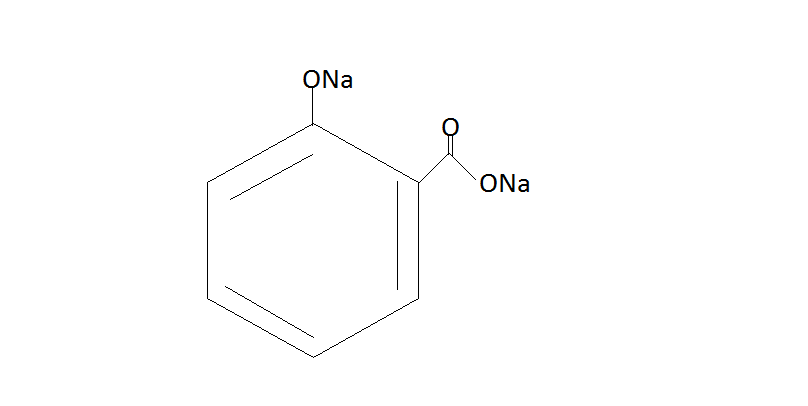
Step $2.$ Now the phenol will react with $C{O_2}{\text{ / NaOH}}$and then we have to do protonation with help of ${H_2}S{O_4}$. The phenol reacts with $C{O_2}{\text{ / NaOH}}$ under pressure which will give us sodium salt or salicylic acid. Then the protonation of this salt yields us salicylic acid.
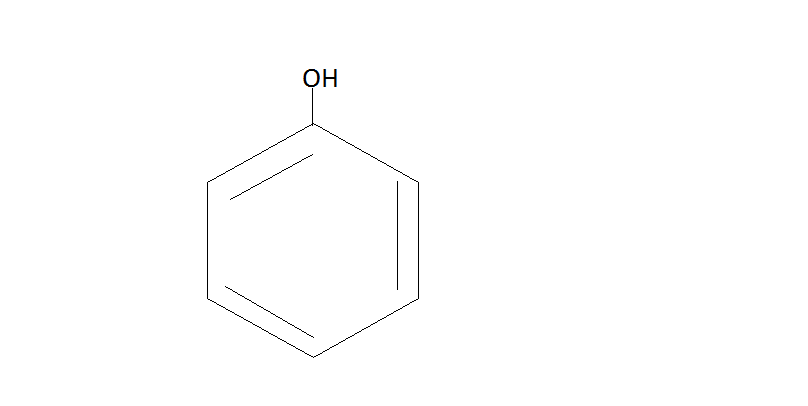 $\xrightarrow{{C{O_2}{\text{ / NaOH}}}}$
$\xrightarrow{{C{O_2}{\text{ / NaOH}}}}$
 $\xrightarrow{{{H_2}S{O_4}}}$
$\xrightarrow{{{H_2}S{O_4}}}$
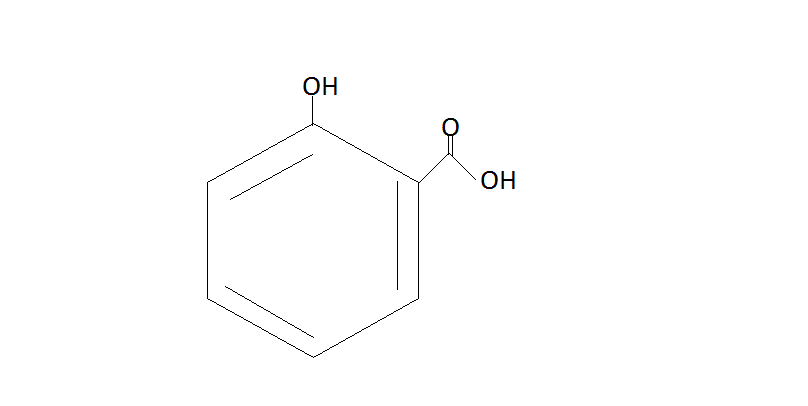
Salicylic acid
Thus we get Salicylic acid from Benzene by using these steps. This reaction is also known as the Kolbe Schmitt Reaction. It is also known as beta hydroxy acid.
Note:
The reaction takes place at high pressure because of the stability of benzene. For protonation we use ${H_2}S{O_4}$ which gives ${H^ + }$. It is preferable to get the hydroxyl group first on the ring because if the carboxyl group is attached first then the ring becomes meta directing in nature. Hence salicylic acid cannot be formed in this way. Thus the hydroxyl group is added first since it is ${\text{o - p}}$activating group. Thus we get salicylic acid as our product.
Complete answer:
Benzene can be converted into Salicylic acid using the following steps :
Step $1.$We will first convert Benzene to phenol. There are many through which it can be converted into phenol. One of them is mentioned below:



Benzene Benzene Sulfonic acid Phenol
Here Benzene reacts with sulfuric acid to form Benzene Sulfonic acid. Then this acid further reacts with $NaOH$at ${200^ \circ }C$ . This will give us phenol.
Step $2.$ Now the phenol will react with $C{O_2}{\text{ / NaOH}}$and then we have to do protonation with help of ${H_2}S{O_4}$. The phenol reacts with $C{O_2}{\text{ / NaOH}}$ under pressure which will give us sodium salt or salicylic acid. Then the protonation of this salt yields us salicylic acid.




Salicylic acid
Thus we get Salicylic acid from Benzene by using these steps. This reaction is also known as the Kolbe Schmitt Reaction. It is also known as beta hydroxy acid.
Note:
The reaction takes place at high pressure because of the stability of benzene. For protonation we use ${H_2}S{O_4}$ which gives ${H^ + }$. It is preferable to get the hydroxyl group first on the ring because if the carboxyl group is attached first then the ring becomes meta directing in nature. Hence salicylic acid cannot be formed in this way. Thus the hydroxyl group is added first since it is ${\text{o - p}}$activating group. Thus we get salicylic acid as our product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




