
Benzene can be conveniently converted into n-propyl benzene by:
A.Friedel-craft alkylation with n-propyl chloride.
B.Friedel-Craft acylation with propionyl chloride followed by Wolff-Kishner reduction.
C.Friedel-Craft acylation with propionyl chloride followed by catalytic hydrogenation.
D.Friedel-Craft acylation with propionyl chloride followed by reduction with \[LiAl{{H}_{4}}\]
Answer
597.9k+ views
Hint: Preparation of n-propylbenzene from Benzene is a two-step process. We cannot prepare n-propylbenzene from benzene in a single step. In the first step benzene reacts with propionyl chloride and then undergoes reduction with hydrazine.
Complete answer:
The preparation of n-propyl benzene from benzene is as follows.
Step-1: Friedel-craft acylation:
Reaction of Benzene with propionyl chloride is called Friedel-craft acylation reaction. Because the acyl group is going to be added to benzene.
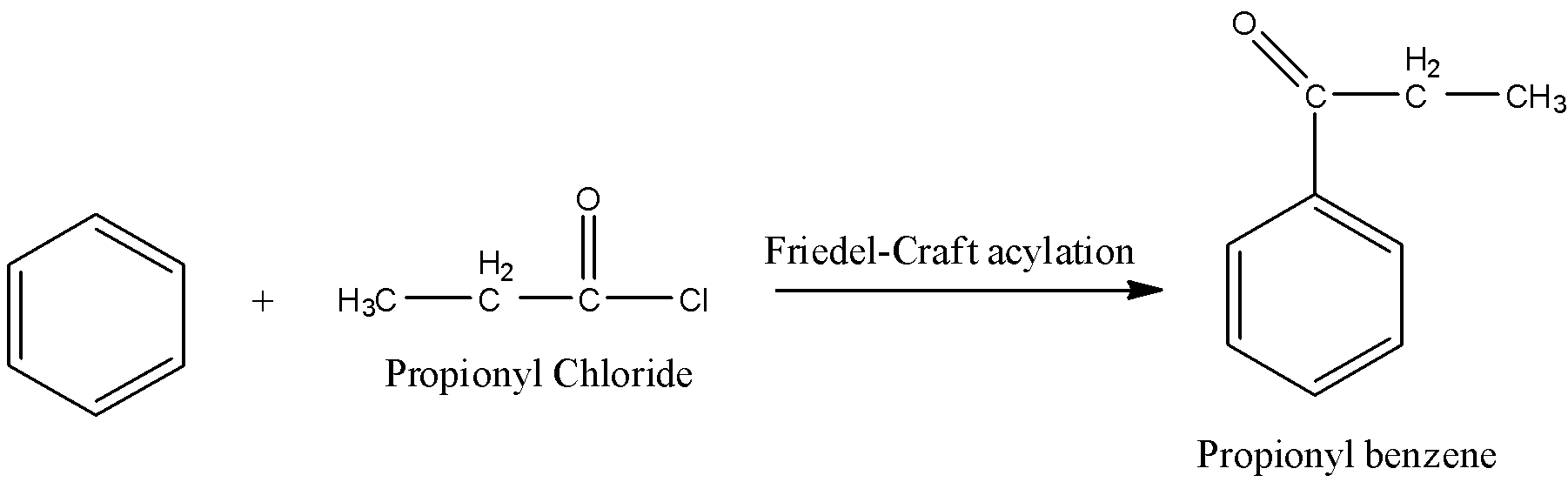
Step-2: Wolff-Kishner reduction:
In this reaction propionyl benzene reacts with hydrazine and forms a hydrazine derivative. This reaction is called Wolff-Kishner reduction.
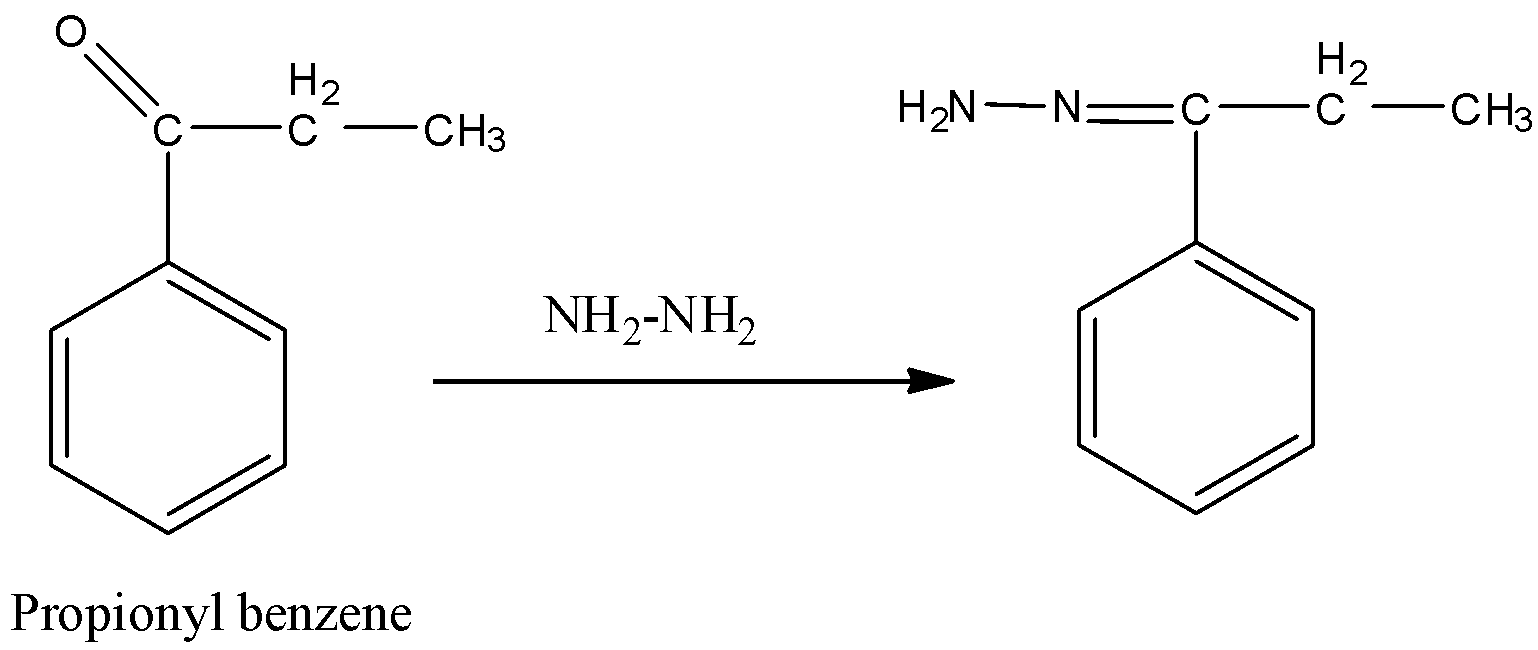
Followed by Wolff-Kishner reduction, reaction with KOH, ethylene glycol forms n-propyl benzene.
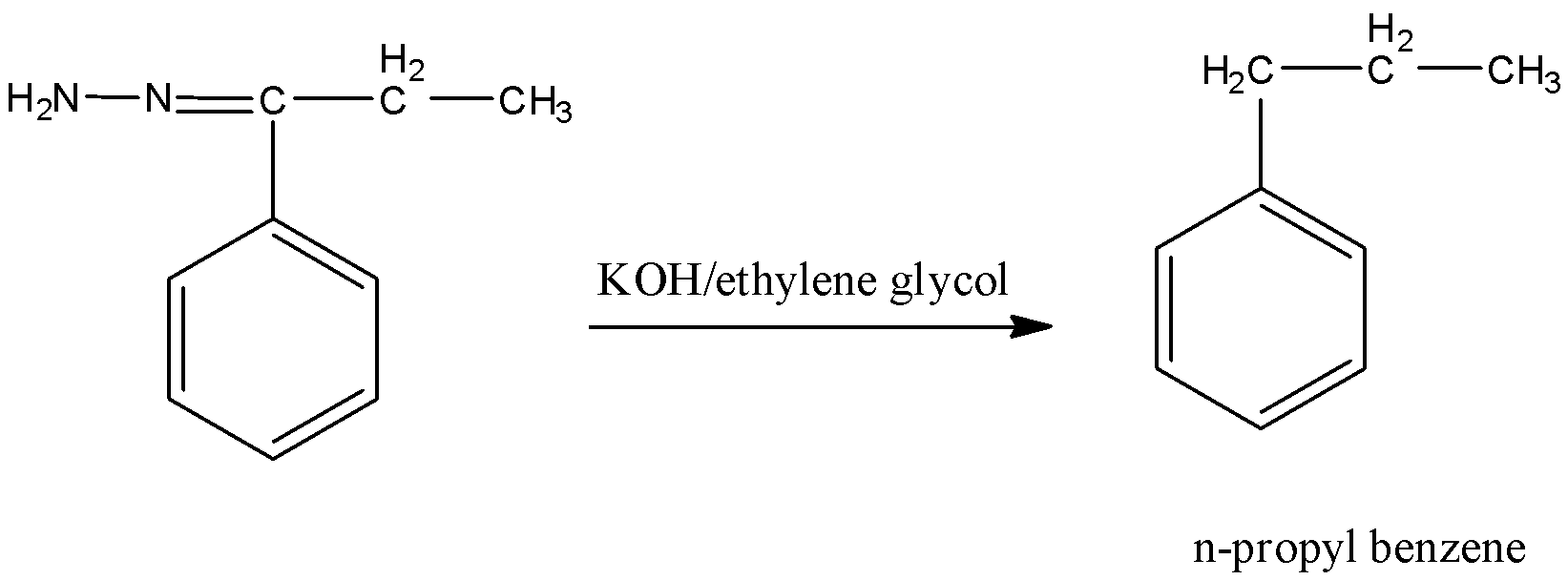
Therefore, the preparation of n-propyl benzene from benzene involves Friedel-Craft acylation with propionyl chloride followed by Wolff-Kishner reduction.
So, the correct option is B.
Note:
We are not supposed to use catalytic hydrogenation or reduction with lithium aluminum hydride (\[LiAl{{H}_{4}}\]) in step-2. Because catalytic hydrogen or reduction with aluminum hydride (\[LiAl{{H}_{4}}\]) may cause reduction on the benzene ring. So, we are not supposed to use these reducing agents in the step-2. Wolff-Kishner reduction is the best way to reduce carbonyl carbon without disturbing the structure of the benzene.
Complete answer:
The preparation of n-propyl benzene from benzene is as follows.
Step-1: Friedel-craft acylation:
Reaction of Benzene with propionyl chloride is called Friedel-craft acylation reaction. Because the acyl group is going to be added to benzene.

Step-2: Wolff-Kishner reduction:
In this reaction propionyl benzene reacts with hydrazine and forms a hydrazine derivative. This reaction is called Wolff-Kishner reduction.

Followed by Wolff-Kishner reduction, reaction with KOH, ethylene glycol forms n-propyl benzene.

Therefore, the preparation of n-propyl benzene from benzene involves Friedel-Craft acylation with propionyl chloride followed by Wolff-Kishner reduction.
So, the correct option is B.
Note:
We are not supposed to use catalytic hydrogenation or reduction with lithium aluminum hydride (\[LiAl{{H}_{4}}\]) in step-2. Because catalytic hydrogen or reduction with aluminum hydride (\[LiAl{{H}_{4}}\]) may cause reduction on the benzene ring. So, we are not supposed to use these reducing agents in the step-2. Wolff-Kishner reduction is the best way to reduce carbonyl carbon without disturbing the structure of the benzene.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




