
What is the basicity of \[{H_3}P{O_3}\] ?
A. 3
B. 2
C. 1
D. None of these
Answer
561k+ views
Hint: We need to know the dependency of the basicity of a compound. The base is a substance that can release $OH^-$ ions when dissolved in water. Similarly, an acid is a substance that can release $H^+$ when dissolved in water. Not all bases and acids have the same ability to donate $OH^-$ and $OH^-$ ions. This ability defines the strength of a base and acid and basicity is a measure of this strength.
Complete step by step answer:
We need to know that the basicity of an acid is the number of $H^+$ ions released, and the basicity of a base is the number of $OH^-$ ions released when dissolved in water. It determines the strength of an acid or base. The basicity of an acid depends on the number of ionizable hydrogen atoms present in the acid. The basicity of a base depends on the number of ionizable hydroxyl groups present in the base.
The given compound is \[{H_3}P{O_3}\] . Clearly, it is an acid as there are no hydroxyl groups present. We now write the skeletal structure of the acid to understand the location of the hydrogen atoms to determine its basicity.
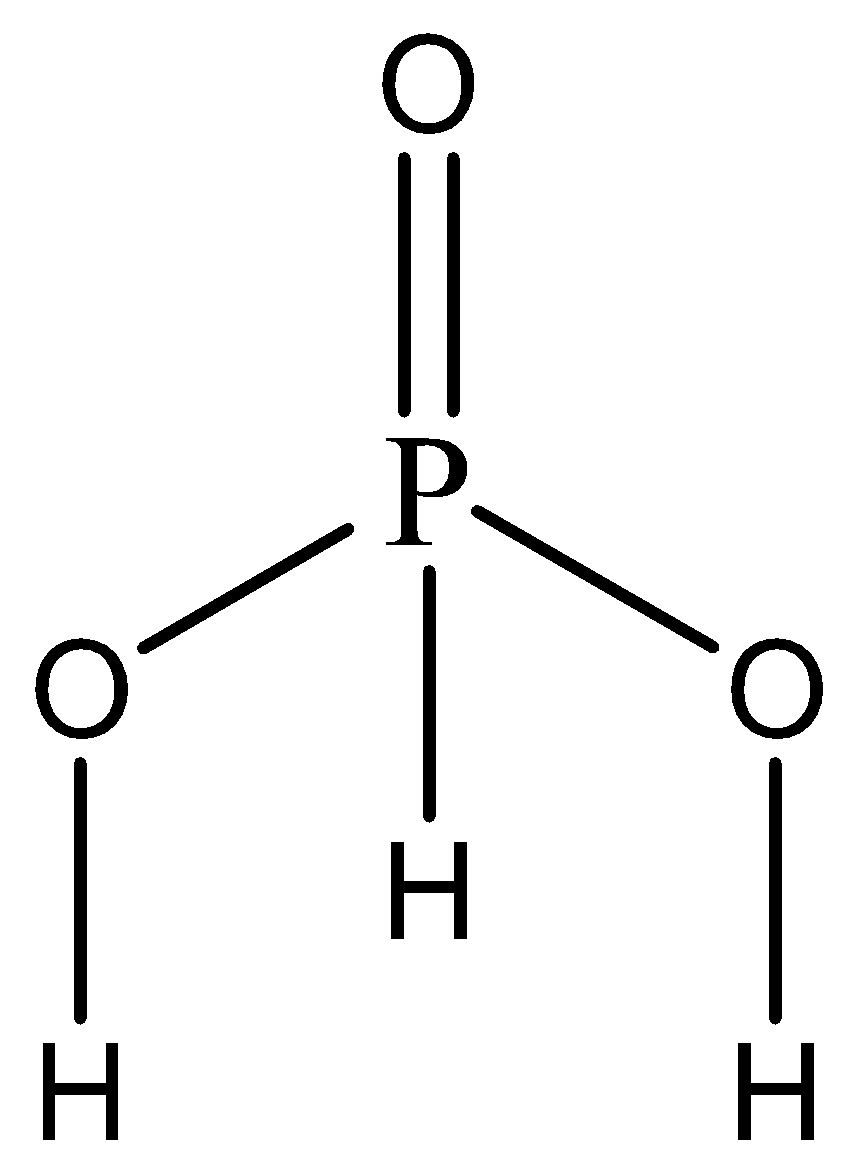
The hydrogen atoms attached directly to the oxygen atoms are ionisable. The hydrogen atom attached to the phosphorus atom is reducing in nature and non-ionisable.
Since there are two ionizable hydrogen groups in \[{H_3}P{O_3}\] , hence the basicity of this acid is 2.
So, the correct answer is Option B.
Note: It must be noted that the basicity of an acid with basicity 2 is also known as dibasic acid. The acids with a single ionizable hydrogen ion are known as monobasic acid and those with three ionizable hydrogen groups are known as tribasic acids. \[{H_3}P{O_3}\] is a strong reducing agent and is also known as phosphorus acid or ortho phosphorous acid.
Complete step by step answer:
We need to know that the basicity of an acid is the number of $H^+$ ions released, and the basicity of a base is the number of $OH^-$ ions released when dissolved in water. It determines the strength of an acid or base. The basicity of an acid depends on the number of ionizable hydrogen atoms present in the acid. The basicity of a base depends on the number of ionizable hydroxyl groups present in the base.
The given compound is \[{H_3}P{O_3}\] . Clearly, it is an acid as there are no hydroxyl groups present. We now write the skeletal structure of the acid to understand the location of the hydrogen atoms to determine its basicity.

The hydrogen atoms attached directly to the oxygen atoms are ionisable. The hydrogen atom attached to the phosphorus atom is reducing in nature and non-ionisable.
Since there are two ionizable hydrogen groups in \[{H_3}P{O_3}\] , hence the basicity of this acid is 2.
So, the correct answer is Option B.
Note: It must be noted that the basicity of an acid with basicity 2 is also known as dibasic acid. The acids with a single ionizable hydrogen ion are known as monobasic acid and those with three ionizable hydrogen groups are known as tribasic acids. \[{H_3}P{O_3}\] is a strong reducing agent and is also known as phosphorus acid or ortho phosphorous acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




