
Base strength of ${\text{(i)}}{{\text{H}}_{\text{3}}}{\text{CC}}{{\text{H}}_{\text{2}}}$ ${\text{(ii)}}{{\text{H}}_{\text{2}}}{\text{C = CH}}$
(iii)
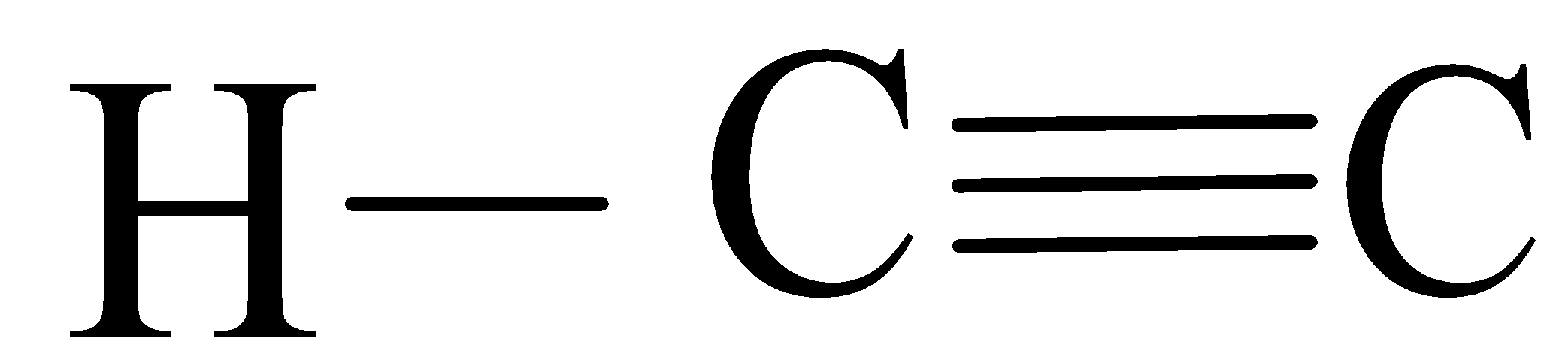
A) (i) > (iii) > (ii)
B) (i) > (ii) > (iii)
C) (ii) > (i) > (iii)
D) (iii) > (ii) > (i)
Answer
564.6k+ views
Hint: The strengths of acids and bases in aqueous solutions can be determined by their acid or base ionization constants. Stronger acids form weaker conjugate bases and weaker acids from strong conjugate bases. The conjugate bases are weaker bases than water because strong acids are ionized in aqueous solution completely.
Complete step by step answer:
Stronger the acid, weaker is its conjugate base. The strength of these conjugate acids is in the order:
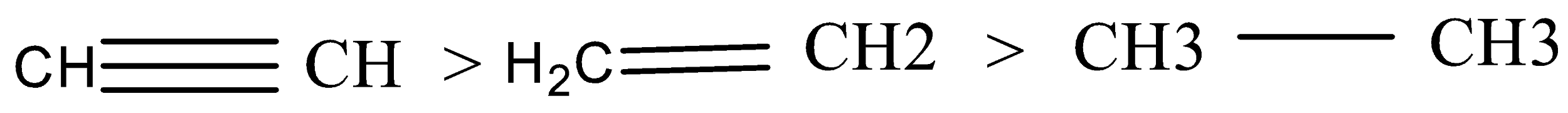
Acidic character:
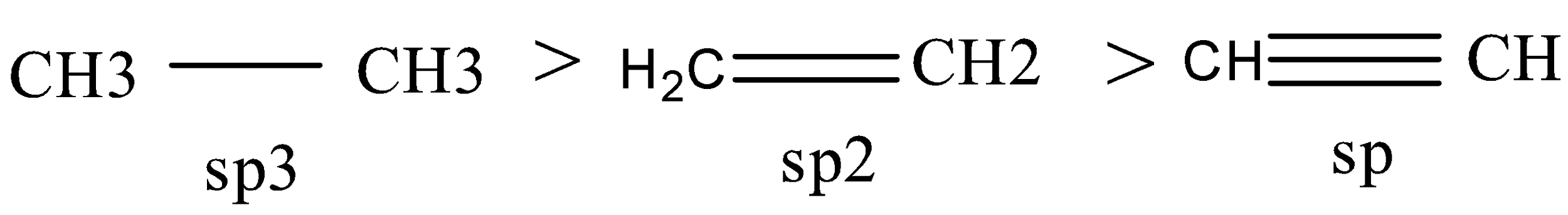
${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised and ${\text{CH}}$ has single bond and there’s no polarity.
${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised is less acidic compared to ${\text{sp}}$ hybridization.
${\text{sp}}$ hybridised and it can easily give ${{\text{H}}^{\text{ + }}}$. hence more acidic
Therefore, the correct order of their conjugate base is:
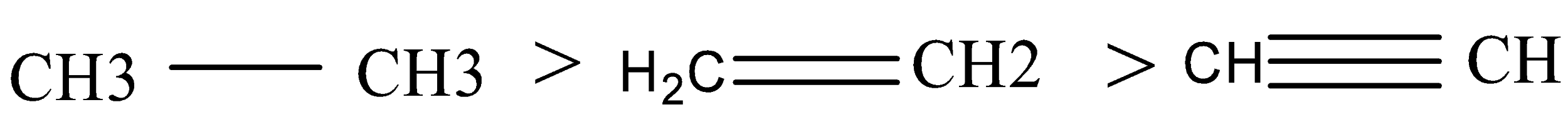
Thus, the correct option is B .
Additional information:
Weak acids are partially ionised as their conjugate bases are strong enough to compete successfully with water for possession of protons. Strong bases react with water to form hydroxide ions. Weak bases give small amounts of hydroxide ion.
The strengths of the binary acids increase from left to right across a periodic table ${\text{(C}}{{\text{H}}_{\text{4}}}{\text{ < N}}{{\text{H}}_{\text{3}}}{\text{ < }}{{\text{H}}_{\text{2}}}{\text{O < HF)}}$ and they increase down a group ${\text{(HF < HCL < HBr < HI)}}$
Note: If a molecule is a strong acid, then it is a weak base and If it is a weak acid, then it is a strong base.
The strength of a base is determined by
The electronegativity of an atom. Less electronegativity of an atom, the more basic it will be.
By delocalizing the electron density by resonance
As the electronegativity of the central element increases, the strength of oxyacids also increases.
Complete step by step answer:
Stronger the acid, weaker is its conjugate base. The strength of these conjugate acids is in the order:
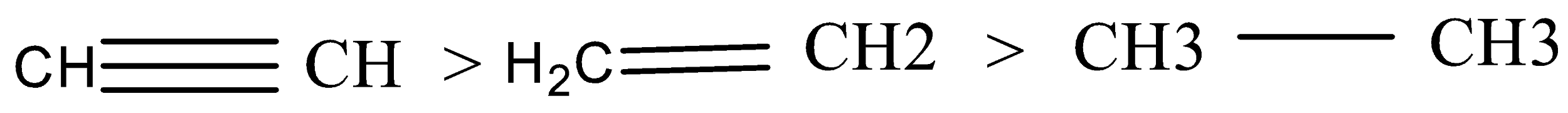
Acidic character:
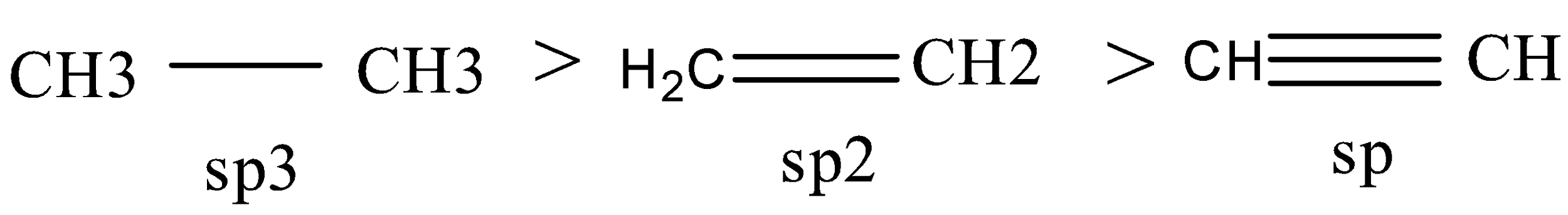
${\text{s}}{{\text{p}}^{\text{3}}}$ hybridised and ${\text{CH}}$ has single bond and there’s no polarity.
${\text{s}}{{\text{p}}^{\text{2}}}$ hybridised is less acidic compared to ${\text{sp}}$ hybridization.
${\text{sp}}$ hybridised and it can easily give ${{\text{H}}^{\text{ + }}}$. hence more acidic
Therefore, the correct order of their conjugate base is:
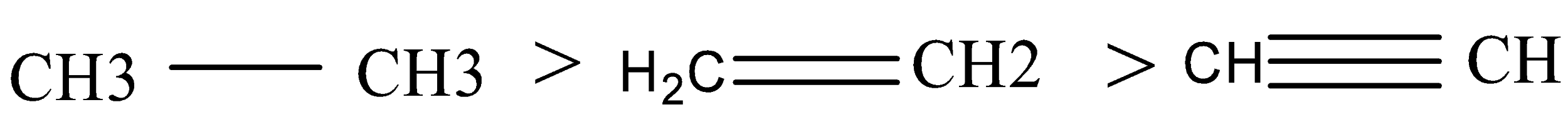
Thus, the correct option is B .
Additional information:
Weak acids are partially ionised as their conjugate bases are strong enough to compete successfully with water for possession of protons. Strong bases react with water to form hydroxide ions. Weak bases give small amounts of hydroxide ion.
The strengths of the binary acids increase from left to right across a periodic table ${\text{(C}}{{\text{H}}_{\text{4}}}{\text{ < N}}{{\text{H}}_{\text{3}}}{\text{ < }}{{\text{H}}_{\text{2}}}{\text{O < HF)}}$ and they increase down a group ${\text{(HF < HCL < HBr < HI)}}$
Note: If a molecule is a strong acid, then it is a weak base and If it is a weak acid, then it is a strong base.
The strength of a base is determined by
The electronegativity of an atom. Less electronegativity of an atom, the more basic it will be.
By delocalizing the electron density by resonance
As the electronegativity of the central element increases, the strength of oxyacids also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




