
Balance the following equation by oxidation number method.
$ {K_2}C{r_2}{O_7} + HCl \to KCl + CrC{l_3} + {H_2}O + C{l_2} $
Answer
537.6k+ views
Hint :The oxidation state or sometimes known as oxidation number, defines the degree of oxidation that an atom possesses in a chemical compound. In other words, oxidation number refers to the charge left on the central atom especially when all the bonding pairs of electrons get broken, with the charge allocated to the most electronegative atom.
Complete Step By Step Answer:
Oxidation number method is generally used to balance the redox reactions. This method works on the principle that the amount of oxidation equals the amount of reduction in the whole chemical reaction. Now using this method, we will balance the given chemical equation stepwise as stated below:
Step 1: To write down the correct molecular formula for each of the reactants and products.
$ {K_2}C{r_2}{O_7} + HCl \to KCl + CrC{l_3} + {H_2}O + C{l_2} $
In the given chemical equation, all molecular formula provided is correct.
Step 2: To identify the elements which change their oxidation number during the execution of reaction. We will write the oxidation numbers of atoms above their chemical symbols on each side of the chemical equation.
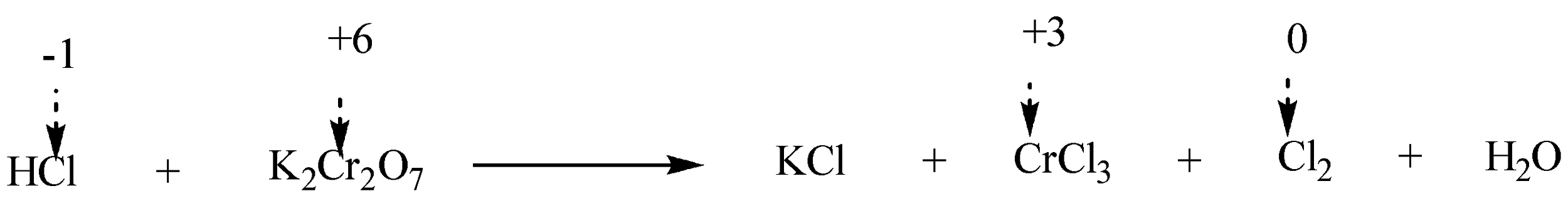
It can be thus visualised that $ Cl $ ion is getting oxidized while $ Cr $ ion is getting reduced.
Step 3: To find out the increase or decrease in oxidation number of each atom. Make them equal by multiplying with a suitable number.
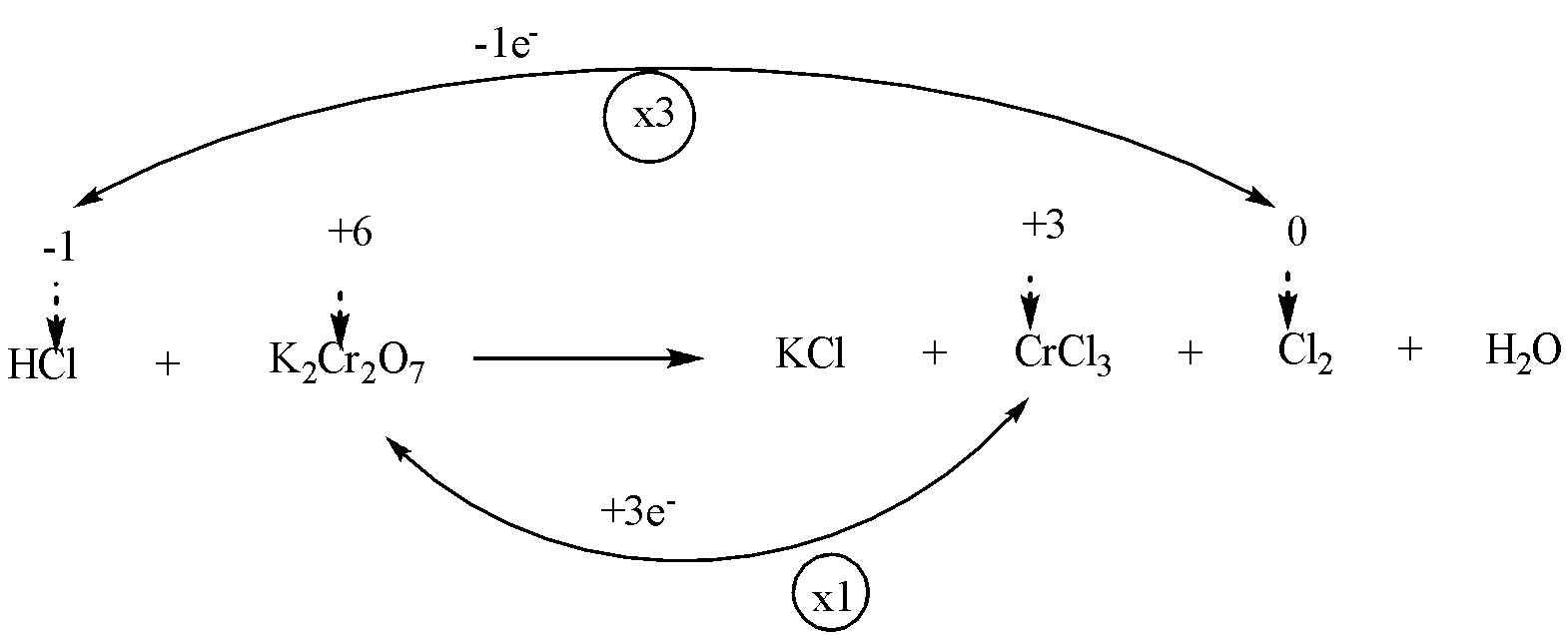
We will multiply $ HCl $ and $ C{l_2} $ by 3 and $ {K_2}C{r_2}{O_7} $ and $ C{l_2} $ by 1.
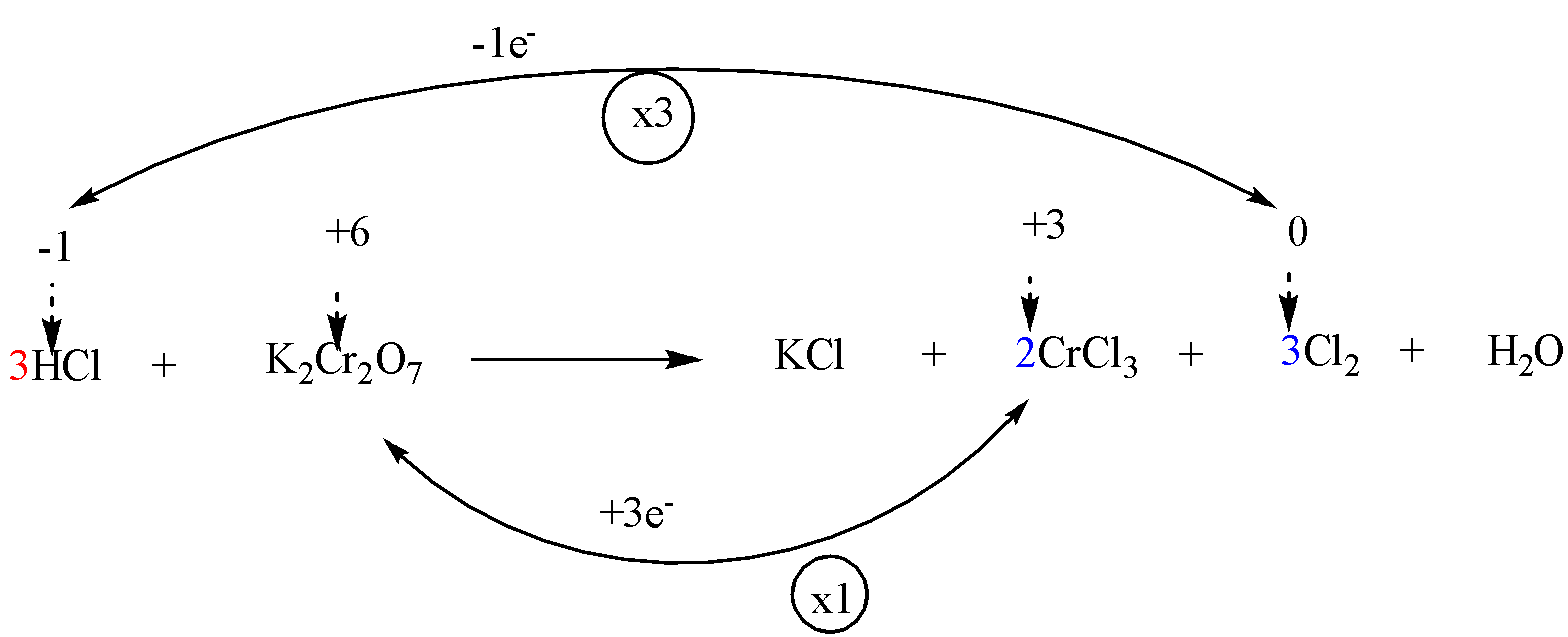
Step 4: To balance the metal ions which don’t change their oxidation number.
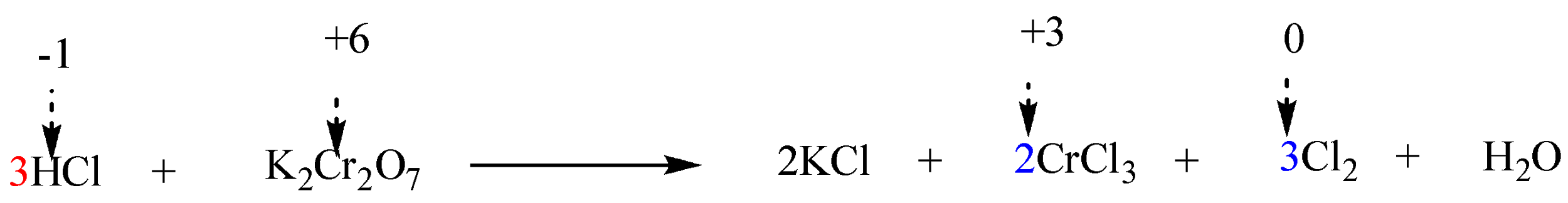
Step 5: Add $ {H^ + } $ (in case of acidic solutions) or $ O{H^ - } $ ions (in case of alkaline solutions) to the chemical reaction on appropriate sides such that total ionic charges of the reactants as well as products are same.
The given reaction in question is in acidic solution (i.e. $ HCl $ ). Thus, $ HCl $ must be added on the side of reactants in order to balance 14 $ Cl $ atoms on the side of products. Therefore, the coefficient of $ HCl $ will be 14.
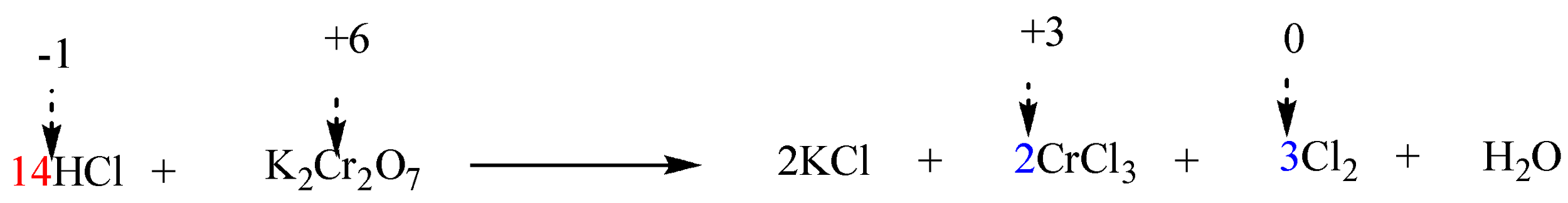
Step 6: Finally balance the number of hydrogen and oxygen atoms in the expression on each side by adding $ {H_2}O $ molecules.
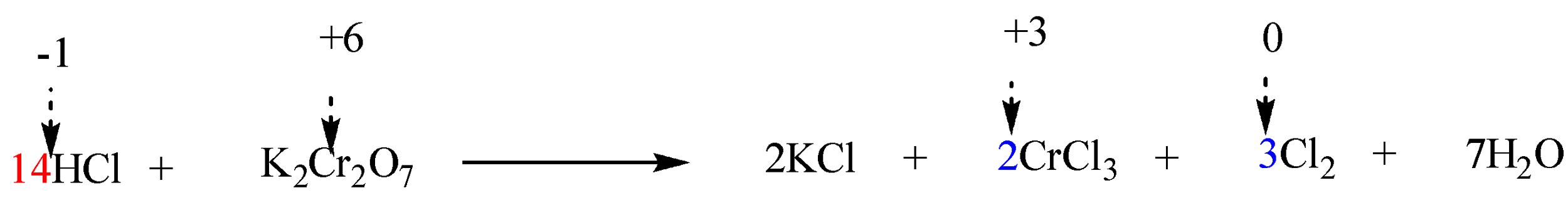
The reaction is now completely balanced.
Note :
A balanced chemical equation simply obeys the law of conservation of mass. Balancing the chemical equations is a significant guiding principle in chemistry. A balanced chemical equation helps you to predict the amount of reactants required and the amount of products formed.
Complete Step By Step Answer:
Oxidation number method is generally used to balance the redox reactions. This method works on the principle that the amount of oxidation equals the amount of reduction in the whole chemical reaction. Now using this method, we will balance the given chemical equation stepwise as stated below:
Step 1: To write down the correct molecular formula for each of the reactants and products.
$ {K_2}C{r_2}{O_7} + HCl \to KCl + CrC{l_3} + {H_2}O + C{l_2} $
In the given chemical equation, all molecular formula provided is correct.
Step 2: To identify the elements which change their oxidation number during the execution of reaction. We will write the oxidation numbers of atoms above their chemical symbols on each side of the chemical equation.

It can be thus visualised that $ Cl $ ion is getting oxidized while $ Cr $ ion is getting reduced.
Step 3: To find out the increase or decrease in oxidation number of each atom. Make them equal by multiplying with a suitable number.

We will multiply $ HCl $ and $ C{l_2} $ by 3 and $ {K_2}C{r_2}{O_7} $ and $ C{l_2} $ by 1.

Step 4: To balance the metal ions which don’t change their oxidation number.

Step 5: Add $ {H^ + } $ (in case of acidic solutions) or $ O{H^ - } $ ions (in case of alkaline solutions) to the chemical reaction on appropriate sides such that total ionic charges of the reactants as well as products are same.
The given reaction in question is in acidic solution (i.e. $ HCl $ ). Thus, $ HCl $ must be added on the side of reactants in order to balance 14 $ Cl $ atoms on the side of products. Therefore, the coefficient of $ HCl $ will be 14.

Step 6: Finally balance the number of hydrogen and oxygen atoms in the expression on each side by adding $ {H_2}O $ molecules.
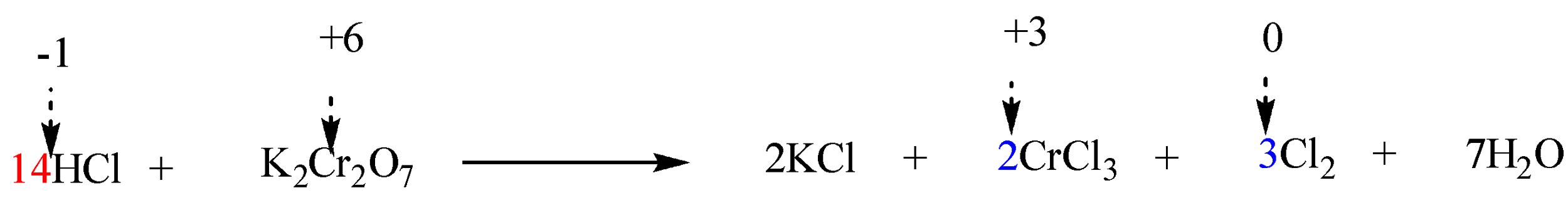
The reaction is now completely balanced.
Note :
A balanced chemical equation simply obeys the law of conservation of mass. Balancing the chemical equations is a significant guiding principle in chemistry. A balanced chemical equation helps you to predict the amount of reactants required and the amount of products formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




