
(B) In the Below Reaction is-
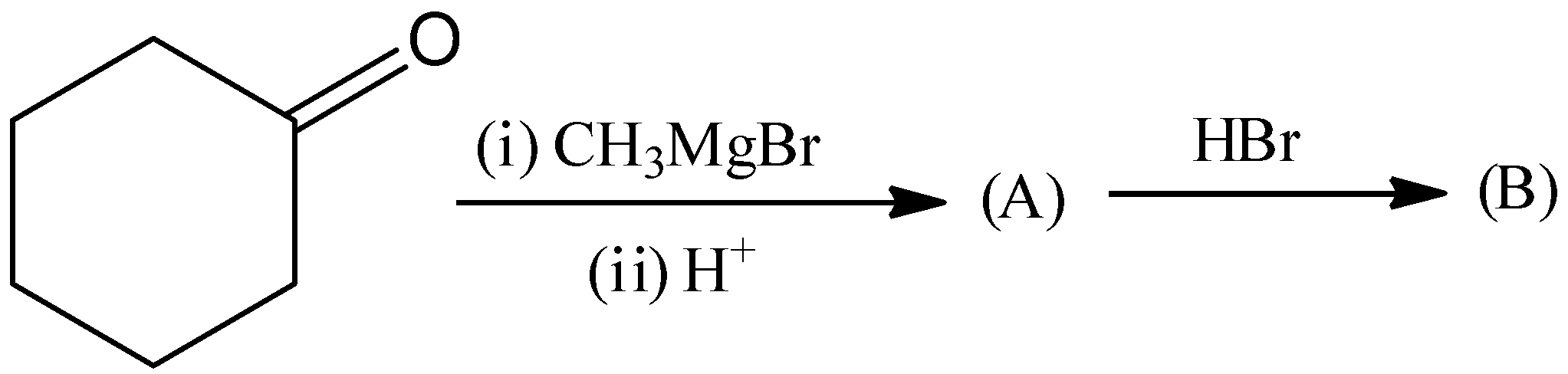
A.
B.
C.
D.
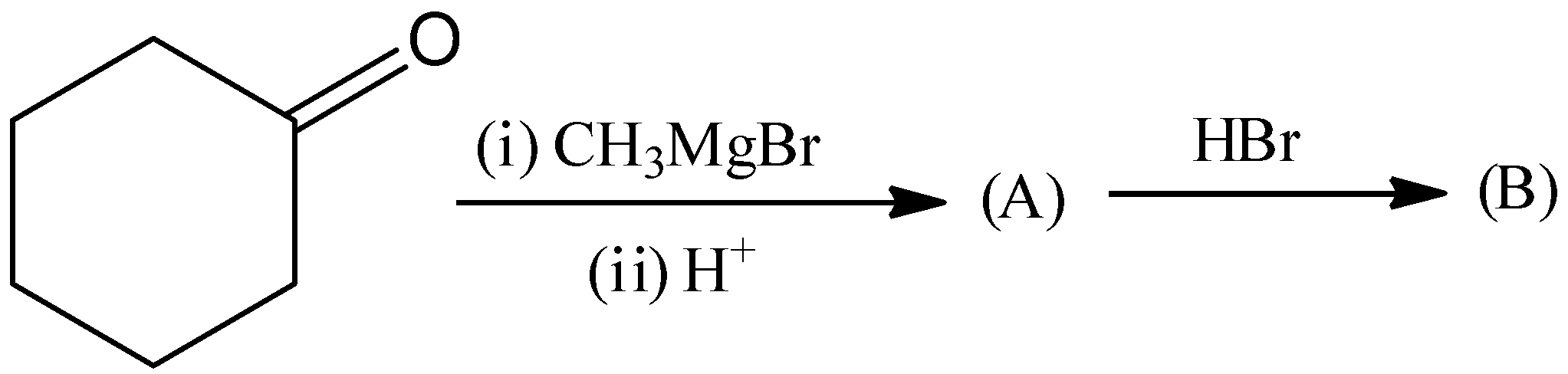




Answer
510.3k+ views
Hint: We must have to know that the cyclohexanone is a chemical compound having the formula, \[{(C{H_2})_5}CO\]. The cyclohexanone is a chemical compound with a functional group, which is ketone. And this is mainly used in paints, acetate cellulose, polymers, copolymers, etc as a solvent. The cyclohexanone is dangerous to our health. Because, it causes headaches, dizziness etc.
Complete answer:
This is not the correct structure of compound (B). Hence, option (A) is incorrect.
Here, the cyclohexanone is reacted with methyl magnesium bromide, (Grignard reagent) and it undergoes hydrolysis and there is a formation of \[1 - \]methylcyclohexan\[ - 1 - \]ol. Let’s see the reaction,
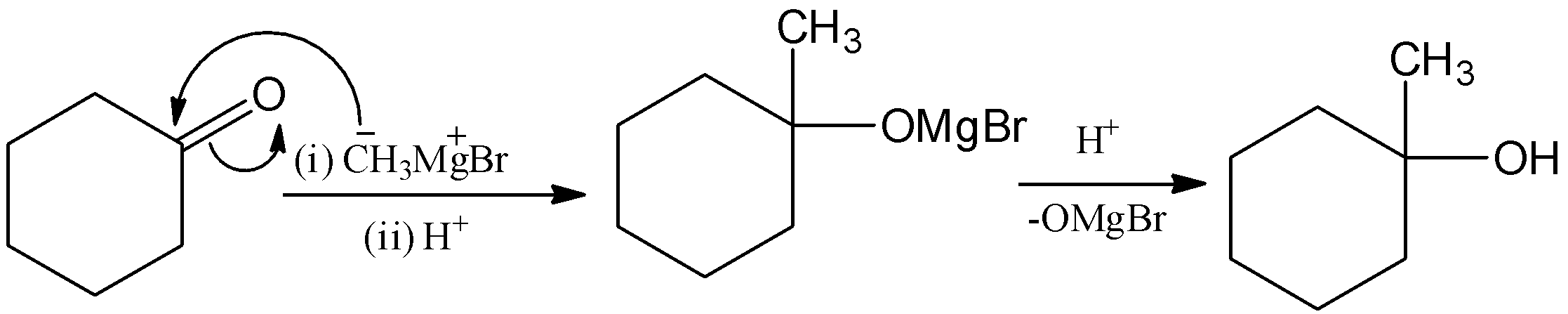
Here, the methyl group will attack the carbon group and form magnesium \[1 - \]methylcyclohexan\[ - 1 - \]olate bromide. And it undergoes hydrolysis, then –OMgBr is replaced by –OH group. Hence, there is a formation of\[1 - \]methylcyclohexan\[ - 1 - \]ol.
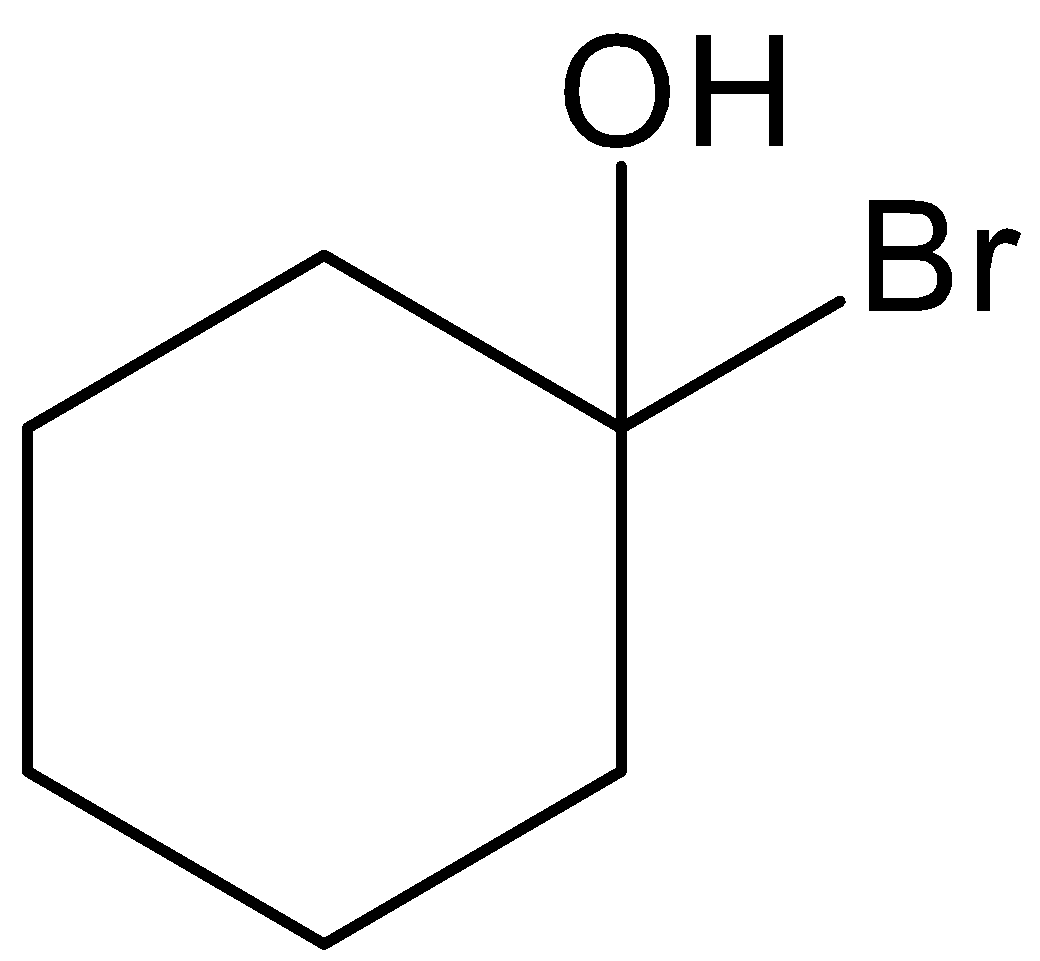
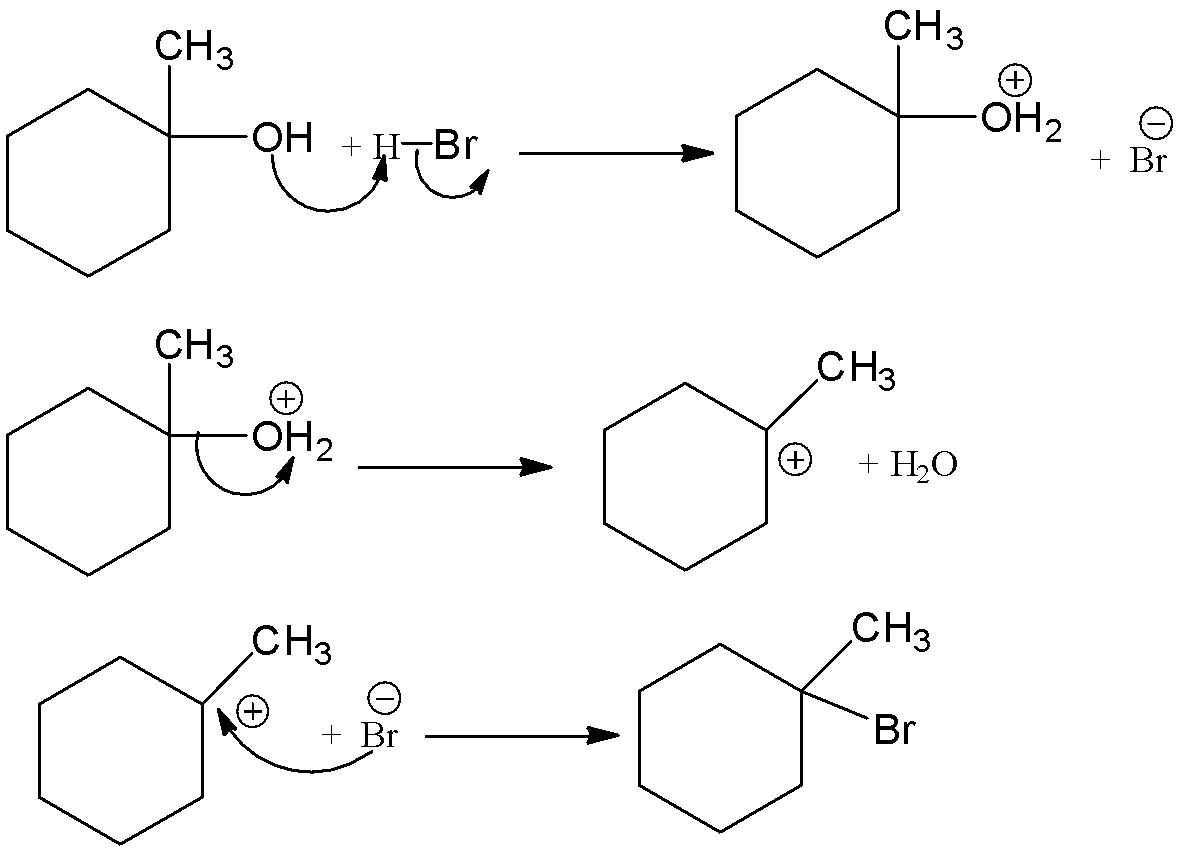
This \[1 - \]methylcyclohexan\[ - 1 - \]ol is reacted with hydrogen bromide and there is a formation of 1-bromo-1-methylcyclohexane. Let’s see the reaction with mechanism,
Thus, overall the reaction can be written as,
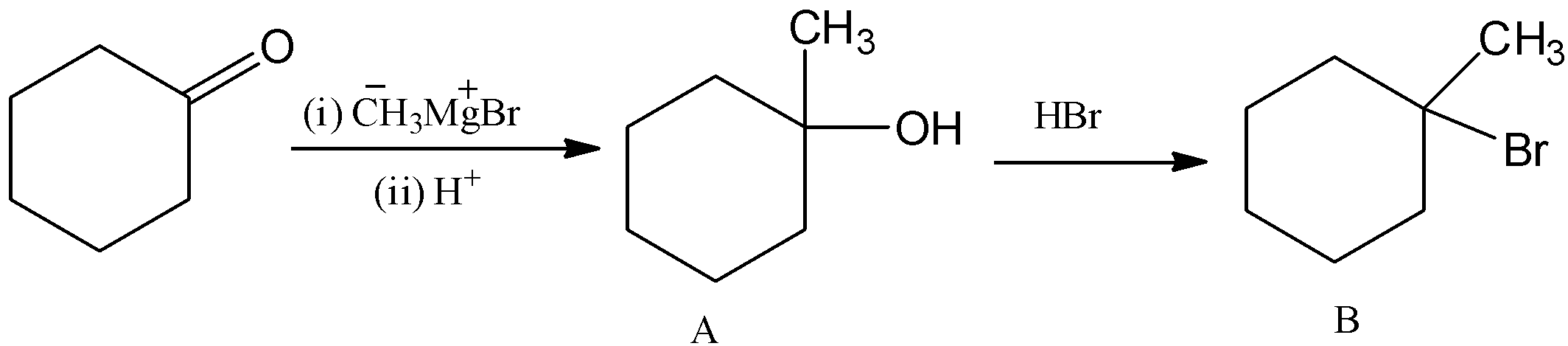
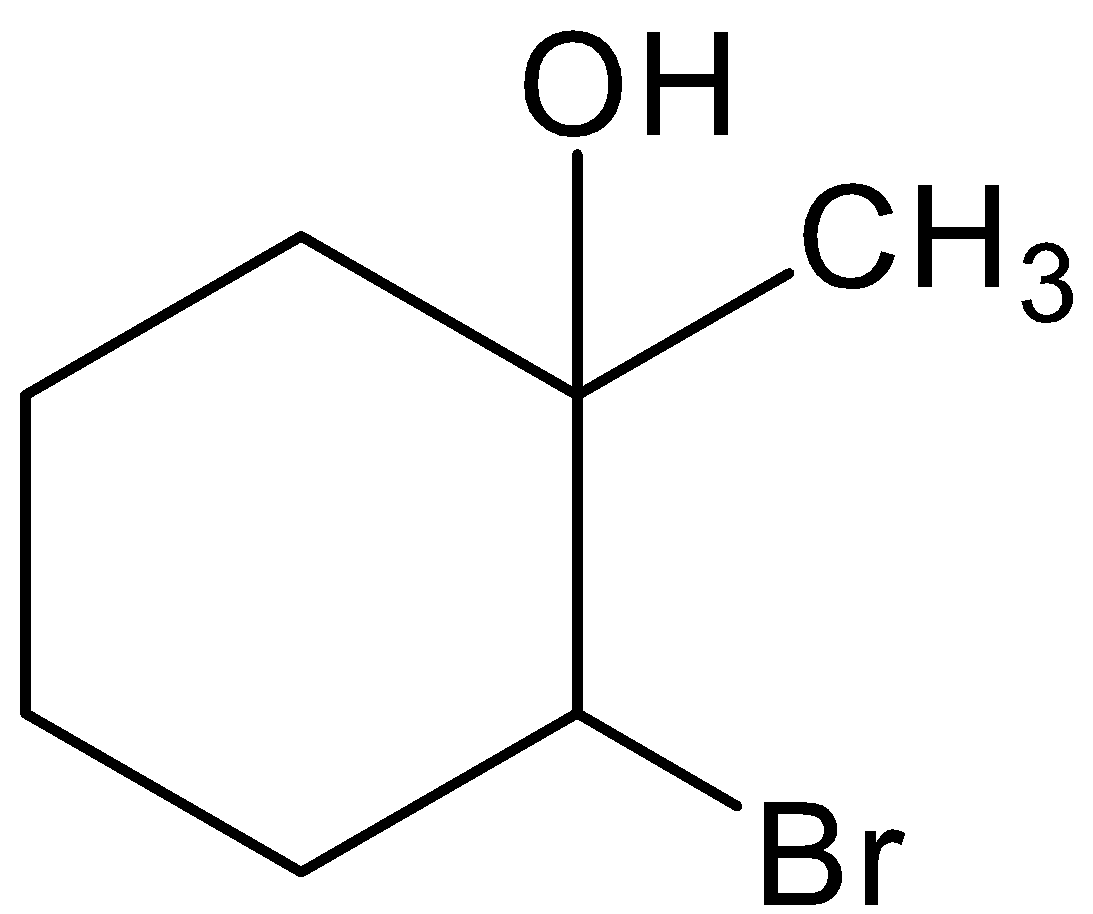
Hence, option (B) is correct.
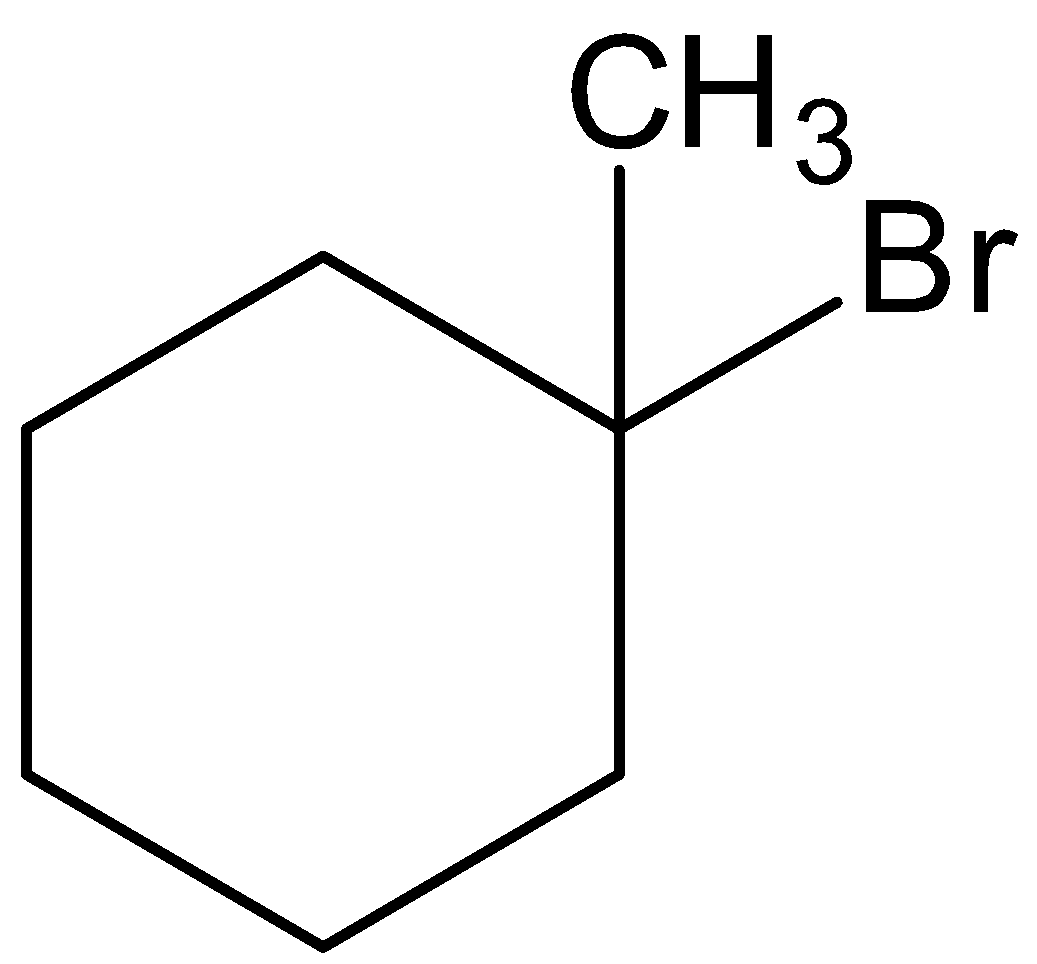
This is not the correct structure of B. option (C) is incorrect.
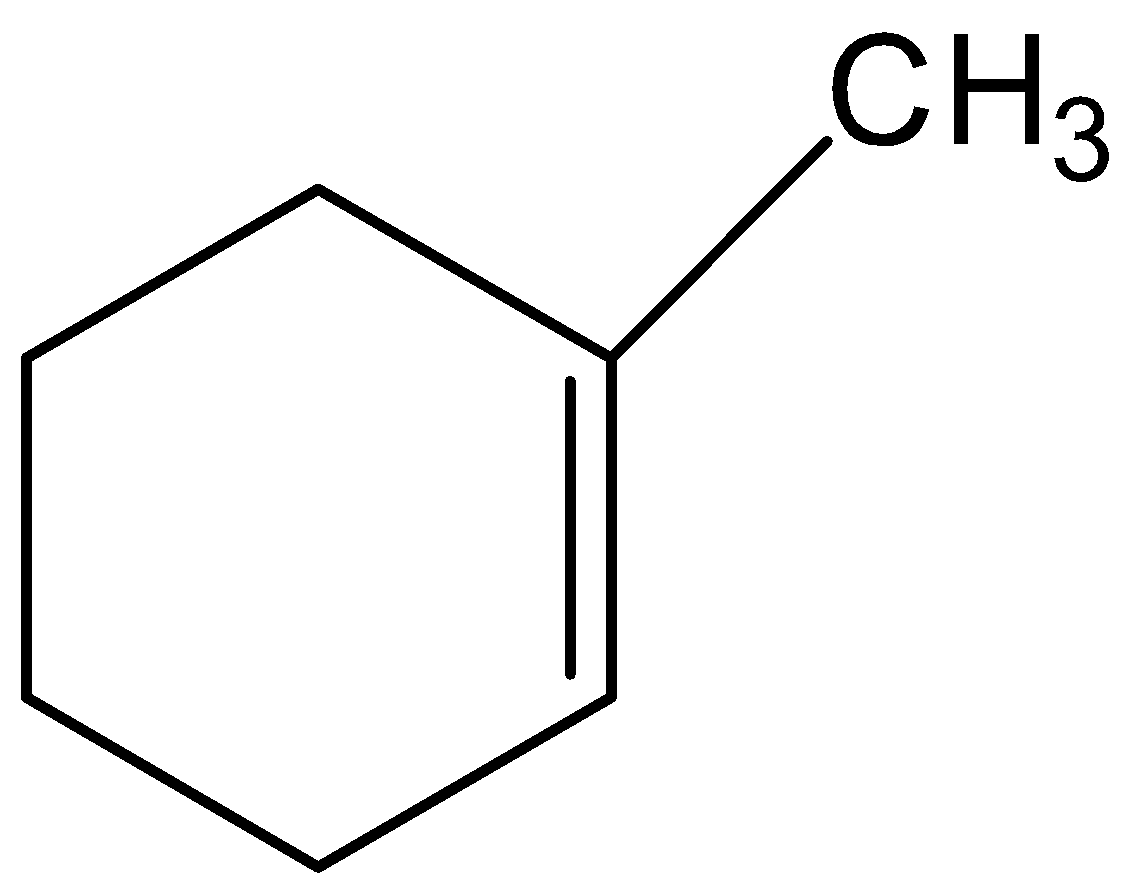
The given compound is not the correct structure of compound B. Hence, option (B) is incorrect.
Hence, the option (B) is correct.
Note:
Here, the cyclohexanone is reacted with Grignard reagent and it undergoes the hybridization reaction. And the Grignard reagent acts as nucleophiles. Here, the cyclohexanone is a ketone. And when the ketone is reacted with Grignard reagent, the carbonyl group is converted into alcohol. This alcohol is treated with hydrogen bromide, and it undergoes the nucleophilic substitution reaction.
Complete answer:
This is not the correct structure of compound (B). Hence, option (A) is incorrect.
Here, the cyclohexanone is reacted with methyl magnesium bromide, (Grignard reagent) and it undergoes hydrolysis and there is a formation of \[1 - \]methylcyclohexan\[ - 1 - \]ol. Let’s see the reaction,

Here, the methyl group will attack the carbon group and form magnesium \[1 - \]methylcyclohexan\[ - 1 - \]olate bromide. And it undergoes hydrolysis, then –OMgBr is replaced by –OH group. Hence, there is a formation of\[1 - \]methylcyclohexan\[ - 1 - \]ol.
This \[1 - \]methylcyclohexan\[ - 1 - \]ol is reacted with hydrogen bromide and there is a formation of 1-bromo-1-methylcyclohexane. Let’s see the reaction with mechanism,

Thus, overall the reaction can be written as,

Hence, option (B) is correct.
This is not the correct structure of B. option (C) is incorrect.
The given compound is not the correct structure of compound B. Hence, option (B) is incorrect.
Hence, the option (B) is correct.
Note:
Here, the cyclohexanone is reacted with Grignard reagent and it undergoes the hybridization reaction. And the Grignard reagent acts as nucleophiles. Here, the cyclohexanone is a ketone. And when the ketone is reacted with Grignard reagent, the carbonyl group is converted into alcohol. This alcohol is treated with hydrogen bromide, and it undergoes the nucleophilic substitution reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




