
AZT stands for:
A. azidothymidine
B. azido thyroxine
C. azide thiol
D. none
Answer
581.7k+ views
Hint: The substance is a medicine and is an antiviral medication. It is used to prevent diseases like HIV or AIDS.
Complete answer
AZT stands for azidothymidine. It is also known as Zidovudine(ZDV). It is generally used to prevent HIV and AIDS and is generally recommended by other antiviral medicines. This medicine can either be taken in by the mouth, or injecting into the veins. It has the chemical formula \[{{C}_{10}}{{H}_{13}}{{N}_{5}}{{O}_{4}}\], and has a molar mass of \[267.2\text{ }g/mol\]. AZT can crystallize into a monoclinic salt structure , and form a hydrogen-nitrogen-oxygen bonded network of dimers where pairing takes up at the base. Although it is used as a medicine, it has some side effects, which include nausea, vomiting, heartburn, headache, less sleep and loss of appetite. There are very few allergic reactions to this compound.
The structure of AZT is:
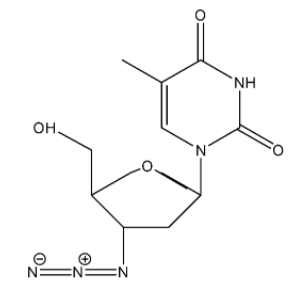
From the structure, we can see that there are two rings present. Stability of a compound depends on the number of rings it has. More rings indicate a more stable compound. AZT is a very old drug and it was approved about 30 years ago. Its place of metabolism is the liver and has a half-life of 0.5 to 3 hours in the human body.
Note:
AZT was the first drug to treat the infection of HIV and was first developed in the year 1984 as a potential cancer therapy by the National Cancer Institute. The structure is not that important, it is better to know the full form, the compound formula and some of the uses, in case if it comes in the exam.
Complete answer
AZT stands for azidothymidine. It is also known as Zidovudine(ZDV). It is generally used to prevent HIV and AIDS and is generally recommended by other antiviral medicines. This medicine can either be taken in by the mouth, or injecting into the veins. It has the chemical formula \[{{C}_{10}}{{H}_{13}}{{N}_{5}}{{O}_{4}}\], and has a molar mass of \[267.2\text{ }g/mol\]. AZT can crystallize into a monoclinic salt structure , and form a hydrogen-nitrogen-oxygen bonded network of dimers where pairing takes up at the base. Although it is used as a medicine, it has some side effects, which include nausea, vomiting, heartburn, headache, less sleep and loss of appetite. There are very few allergic reactions to this compound.
The structure of AZT is:

From the structure, we can see that there are two rings present. Stability of a compound depends on the number of rings it has. More rings indicate a more stable compound. AZT is a very old drug and it was approved about 30 years ago. Its place of metabolism is the liver and has a half-life of 0.5 to 3 hours in the human body.
Note:
AZT was the first drug to treat the infection of HIV and was first developed in the year 1984 as a potential cancer therapy by the National Cancer Institute. The structure is not that important, it is better to know the full form, the compound formula and some of the uses, in case if it comes in the exam.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




