
A.What is the Lewis structure for \[Se{F_4}\] ?
B.What is its electron geometry?
C.What is its molecular geometry?
D.What is its hybridization?
E.How would you classify it in the AXE system?
F.What are the ideal bond angles?
Answer
494.4k+ views
Hint: Selenium is a chemical element belong to oxygen family due to the less electronegativity compared to the fluorine, it involves in the bond formation with fluorine to from a compound named as selenium tetrafluoride with the molecular formula of \[Se{F_4}\] . The electron geometry is trigonal bipyramidal.
Complete answer:
Selenium has six valence electrons and fluorine is one electron deficient to attain inert gas configuration. Out of six electrons, four electrons were involved in bond formation with fluorine. Remaining two electrons will exist as lone pairs on selenium.
The Lewis structure of selenium tetrafluoride is
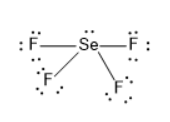
The electron geometry is trigonal bipyramidal.
The molecular geometry is see-saw due to the presence of one lone pair of electrons.
The hybridization is \[s{p^3}d\]
Due to the presence of one metal atom, and four halide atoms and one lone pair of electrons it can be considered as \[A{X_4}E\]
The ideal bond angles in selenium tetrafluoride are \[{102^0}\] and \[{173^0}\] where the equatorial -equatorial bond angle is less than \[{120^0}\]
Note:
Due to the presence of six valence electrons and four fluorine atoms, it is involved in trigonal bipyramidal shape. Due to the presence of lone pairs the molecular geometry is see-saw whereas the electron geometry is trigonal bipyramidal. They both are different in this molecule.
Complete answer:
Selenium has six valence electrons and fluorine is one electron deficient to attain inert gas configuration. Out of six electrons, four electrons were involved in bond formation with fluorine. Remaining two electrons will exist as lone pairs on selenium.
The Lewis structure of selenium tetrafluoride is

The electron geometry is trigonal bipyramidal.
The molecular geometry is see-saw due to the presence of one lone pair of electrons.
The hybridization is \[s{p^3}d\]
Due to the presence of one metal atom, and four halide atoms and one lone pair of electrons it can be considered as \[A{X_4}E\]
The ideal bond angles in selenium tetrafluoride are \[{102^0}\] and \[{173^0}\] where the equatorial -equatorial bond angle is less than \[{120^0}\]
Note:
Due to the presence of six valence electrons and four fluorine atoms, it is involved in trigonal bipyramidal shape. Due to the presence of lone pairs the molecular geometry is see-saw whereas the electron geometry is trigonal bipyramidal. They both are different in this molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




