
How many atom(s) of $S\left( {C{H_2}} \right){F_4}$ may lie in the axial plane?
Answer
589.5k+ views
Hint: $VSEPR$ theory plays an important role in predicting the shape, geometry and hybridization of a molecule. $VSEPR$ theory is valence shell electron pair repulsion theory. Axial bonds are vertical while equatorial bonds are horizontal. These bonds are very helpful while explaining the three dimensional structure of the molecule.
Complete step by step answer:
In this question we have to find the atoms that may lie on the axial plane of $S\left( {C{H_2}} \right){F_4}$ molecule. In this question they are asking how many atoms may lie there, so we will take maximum atoms that are possible on the axial plane. Structure of $S\left( {C{H_2}} \right){F_4}$ molecule is:
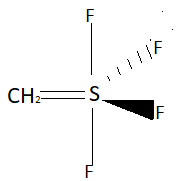
If we take the plane with carbon as the axial plane then there will be three atoms on the axial plane that are carbon and two fluorine atoms represented by bonds other than wedge and dash. Two hydrogen atoms linked with carbon would be out of the axial plane as one hydrogen atom will be on the left side of the axial plane and one hydrogen atom will be on the right of the axial plane. So, taking planes between carbon atoms will contain three atoms in the axial plane.
Now, let’s consider the plane to be passing through fluorine represented by dash. In this case also there will be three atoms on the axial plane and all will be three fluorine atoms. One represented by dash, one which is shown above sulphur (in above structure) and one which is shown below sulphur (in above diagram). So, taking the plane through fluorine represented by a dash will also contain three atoms in the axial plane.
Now, let’s consider the plane to be passing through the fluorine represented by the wedge. In this case also there will be three atoms on the axial plane and all will be three fluorine atoms. One represented by a wedge, one which is shown above sulphur (in above structure) and one which is shown below sulphur (in above diagram). So, taking the plane through the fluorine represented by the wedge will also contain three atoms in the axial plane.
So, there will be three atoms on the axial plane in $S\left( {C{H_2}} \right){F_4}$ molecule.
Note:
Do not include hydrogen atoms with carbon while taking the plane through the carbon atom. Because the hydrogen atoms associated with carbon are not in the axial plane. One hydrogen atom is on the left of the plane and one is on the right of the plane and hence both are out of the axial plane.
Complete step by step answer:
In this question we have to find the atoms that may lie on the axial plane of $S\left( {C{H_2}} \right){F_4}$ molecule. In this question they are asking how many atoms may lie there, so we will take maximum atoms that are possible on the axial plane. Structure of $S\left( {C{H_2}} \right){F_4}$ molecule is:

If we take the plane with carbon as the axial plane then there will be three atoms on the axial plane that are carbon and two fluorine atoms represented by bonds other than wedge and dash. Two hydrogen atoms linked with carbon would be out of the axial plane as one hydrogen atom will be on the left side of the axial plane and one hydrogen atom will be on the right of the axial plane. So, taking planes between carbon atoms will contain three atoms in the axial plane.
Now, let’s consider the plane to be passing through fluorine represented by dash. In this case also there will be three atoms on the axial plane and all will be three fluorine atoms. One represented by dash, one which is shown above sulphur (in above structure) and one which is shown below sulphur (in above diagram). So, taking the plane through fluorine represented by a dash will also contain three atoms in the axial plane.
Now, let’s consider the plane to be passing through the fluorine represented by the wedge. In this case also there will be three atoms on the axial plane and all will be three fluorine atoms. One represented by a wedge, one which is shown above sulphur (in above structure) and one which is shown below sulphur (in above diagram). So, taking the plane through the fluorine represented by the wedge will also contain three atoms in the axial plane.
So, there will be three atoms on the axial plane in $S\left( {C{H_2}} \right){F_4}$ molecule.
Note:
Do not include hydrogen atoms with carbon while taking the plane through the carbon atom. Because the hydrogen atoms associated with carbon are not in the axial plane. One hydrogen atom is on the left of the plane and one is on the right of the plane and hence both are out of the axial plane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




