
How many atom(s) of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ may lie in the equatorial plane?
Answer
585.3k+ views
Hint: The vertical bonds are known as the axial bonds whereas the horizontal bonds are known as the equatorial bonds. The axial bonds are parallel to the plane of the molecule while the equatorial bonds are perpendicular to the plane.
Complete step by step answer:
-The structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is as follows:
The central atom is the sulfur atom. The four fluorine groups are attached to the central sulfur atom by single bond. And the methylene group is attached to the central sulfur atom by a double bond. Thus, the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is,
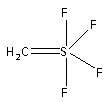
-In the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ the one methylene group and two fluorine atoms are attached in such a manner that they form a trigonal structure. The remaining two fluorine atoms are attached like the two pyramids. Thus, the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is trigonal bipyramidal.
-In trigonal bipyramidal structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$, the two fluorine atoms are placed vertically and are parallel to the plane of the molecule. Of the other two fluorine atoms, one fluorine atom lies to the right side of the plane while the other lies to the left side of the plane. The methylene group is attached horizontally and is perpendicular to the plane of the molecule.
Thus, ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ has one atom in the equatorial plane.
Note: The hydrogen atoms are attached to the carbon atom of the methylene group and not to the central sulfur atoms. The hydrogen atoms lie on the right and left sides of the plane. Thus, the hydrogen atoms are neither axial or equatorial.
Complete step by step answer:
-The structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is as follows:
The central atom is the sulfur atom. The four fluorine groups are attached to the central sulfur atom by single bond. And the methylene group is attached to the central sulfur atom by a double bond. Thus, the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is,

-In the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ the one methylene group and two fluorine atoms are attached in such a manner that they form a trigonal structure. The remaining two fluorine atoms are attached like the two pyramids. Thus, the structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ is trigonal bipyramidal.
-In trigonal bipyramidal structure of ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$, the two fluorine atoms are placed vertically and are parallel to the plane of the molecule. Of the other two fluorine atoms, one fluorine atom lies to the right side of the plane while the other lies to the left side of the plane. The methylene group is attached horizontally and is perpendicular to the plane of the molecule.
Thus, ${\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}$ has one atom in the equatorial plane.
Note: The hydrogen atoms are attached to the carbon atom of the methylene group and not to the central sulfur atoms. The hydrogen atoms lie on the right and left sides of the plane. Thus, the hydrogen atoms are neither axial or equatorial.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




