
How do the atoms combine in carbon-dioxide or \[C{O_2}\]?
A.Forming ionic bond
B.Forming covalent bond
C.Forming metallic bond
D.None of these
Answer
579.9k+ views
Hint: Ionic bond is formed when one atom donates its electron to the other atom in the molecule. Covalent bond is formed when valence electrons are shared between the atoms to form a molecule. Metallic bond is formed between two metal atoms in which cations share many delocalized electrons.
Complete step by step answer:
Carbon dioxide is formed by the reaction of carbon and oxygen. The reaction is given as-
$C + {O_2} \to C{O_2}$
In carbon dioxide, the two oxygen atoms are attached to one carbon atom. We know that Carbon has atomic number $6$ and the electronic configuration of Carbon is$1{s^2}2{s^2}2{p^2}$. This means the outermost shell has $4$ electrons which are called valence electrons. So carbon needs four more electrons to complete its octet.
Oxygen has atomic number $8$ and its electronic configuration is written as-$1{s^2}2{s^2}2{p^4}$ .This means that the outermost shell has $6$ valence electrons so one atom of oxygen will need $2$ more electrons to complete its octet. Then $2$ atoms will need four electrons to complete their octet.
In carbon dioxide, both the atoms need four electrons to complete their octet so they share their four electrons between them and covalent is formed between the carbon and oxygen atom. We can understand this through Lewis dot structure in which the dot represents the valence electrons of an atom.
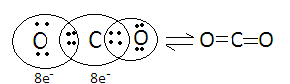
Hence the atoms in carbon dioxide combine forming a covalent bond.
The correct answer is option B
Note:
Carbon dioxide is one of the main greenhouse gases. It is used as following-
-It is used in fire extinguishers as it decreases the concentration of oxygen in the air.
-Liquid carbon dioxide is used as a solvent for lipophilic organic compounds.
-It is used in the water treatment process to stabilize the pH of water.
-It is used as a soldering agent.
-It is used to make many chemical compounds like urea, methanol and carbonates.
Complete step by step answer:
Carbon dioxide is formed by the reaction of carbon and oxygen. The reaction is given as-
$C + {O_2} \to C{O_2}$
In carbon dioxide, the two oxygen atoms are attached to one carbon atom. We know that Carbon has atomic number $6$ and the electronic configuration of Carbon is$1{s^2}2{s^2}2{p^2}$. This means the outermost shell has $4$ electrons which are called valence electrons. So carbon needs four more electrons to complete its octet.
Oxygen has atomic number $8$ and its electronic configuration is written as-$1{s^2}2{s^2}2{p^4}$ .This means that the outermost shell has $6$ valence electrons so one atom of oxygen will need $2$ more electrons to complete its octet. Then $2$ atoms will need four electrons to complete their octet.
In carbon dioxide, both the atoms need four electrons to complete their octet so they share their four electrons between them and covalent is formed between the carbon and oxygen atom. We can understand this through Lewis dot structure in which the dot represents the valence electrons of an atom.
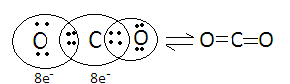
Hence the atoms in carbon dioxide combine forming a covalent bond.
The correct answer is option B
Note:
Carbon dioxide is one of the main greenhouse gases. It is used as following-
-It is used in fire extinguishers as it decreases the concentration of oxygen in the air.
-Liquid carbon dioxide is used as a solvent for lipophilic organic compounds.
-It is used in the water treatment process to stabilize the pH of water.
-It is used as a soldering agent.
-It is used to make many chemical compounds like urea, methanol and carbonates.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




