
Atomicity of oxygen in ozone molecules is _________.
Answer
592.2k+ views
Hint: Atomicity of an element is defined as the total number of atoms present in one molecule of the element. Depending upon the number of atoms present in a molecule, it can be monatomic, diatomic, triatomic, or polyatomic.
Complete answer:
Ozone has the molecular formula ${{O}_{3}}$. Ozone is another form of oxygen element.
We know that the atomicity is the total number of atoms present in one molecule. For example, oxygen is present in the atmosphere as ${{O}_{2}}$. It has two oxygen atoms, therefore, its atomicity is 2.
Similarly, we can determine the atomicity of ozone. One molecule of ozone (${{O}_{3}}$) is composed of three atoms. Thus, the atomicity of oxygen in ozone is 3. In other words, we can say that ozone is a triatomic molecule.
Alternatively, we can also find out the number of oxygen atoms (i.e. atomicity) in ozone from the ratio of molecular mass of ozone to the atomic mass of oxygen., i.e.
\[\text{Atomicity = }\dfrac{\text{molecular}\,\text{mass}\,\text{of}\,{{\text{O}}_{3}}}{\text{atomic}\,\text{mass}\,\text{of}\,\text{O}}\]
Atomic mass of oxygen = 16g
Molecular mass of ${{O}_{3}}$ = $3\times 16$ = 48g
Therefore, on dividing the two, we get atomicity = $\dfrac{48g}{16g}=3$.
Hence, atomicity of oxygen in ozone molecules is 3.
So, the correct answer is “Option D”.
Additional Information: Structure of ozone molecule is explained by resonance.
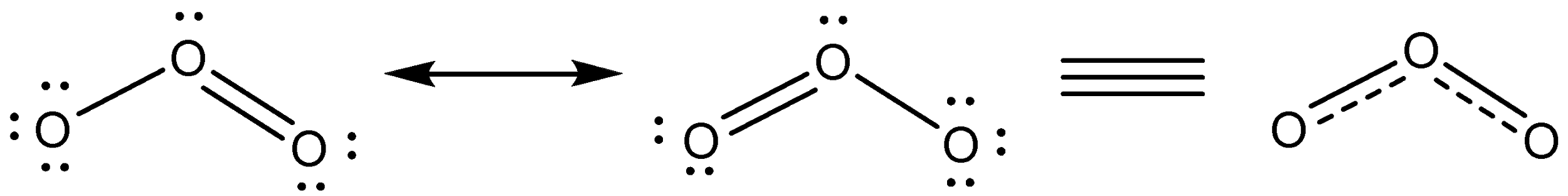
Ozone is found in the upper atmosphere. It is formed by the action of ultraviolet radiation on ${{O}_{2}}$ . The high energy UV radiation splits ${{O}_{2}}$ into oxygen atoms, which react with ${{O}_{2}}$ to form ${{O}_{3}}$.
\[\begin{align}
& {{O}_{2}}\xrightarrow{hv}O+O \\
& O+{{O}_{2}}\to {{O}_{3}} \\
\end{align}\]
Note: Note that ozone is a homonuclear molecule as it contains only one type of atoms. It is easier to count the total number of atoms in homonuclear molecules than in heteronuclear molecules, which contain more than one type of atoms. So in such cases, divide the mass of the molecule by the mass of the atom, of which you want to find the atomicity.
Complete answer:
Ozone has the molecular formula ${{O}_{3}}$. Ozone is another form of oxygen element.
We know that the atomicity is the total number of atoms present in one molecule. For example, oxygen is present in the atmosphere as ${{O}_{2}}$. It has two oxygen atoms, therefore, its atomicity is 2.
Similarly, we can determine the atomicity of ozone. One molecule of ozone (${{O}_{3}}$) is composed of three atoms. Thus, the atomicity of oxygen in ozone is 3. In other words, we can say that ozone is a triatomic molecule.
Alternatively, we can also find out the number of oxygen atoms (i.e. atomicity) in ozone from the ratio of molecular mass of ozone to the atomic mass of oxygen., i.e.
\[\text{Atomicity = }\dfrac{\text{molecular}\,\text{mass}\,\text{of}\,{{\text{O}}_{3}}}{\text{atomic}\,\text{mass}\,\text{of}\,\text{O}}\]
Atomic mass of oxygen = 16g
Molecular mass of ${{O}_{3}}$ = $3\times 16$ = 48g
Therefore, on dividing the two, we get atomicity = $\dfrac{48g}{16g}=3$.
Hence, atomicity of oxygen in ozone molecules is 3.
So, the correct answer is “Option D”.
Additional Information: Structure of ozone molecule is explained by resonance.

Ozone is found in the upper atmosphere. It is formed by the action of ultraviolet radiation on ${{O}_{2}}$ . The high energy UV radiation splits ${{O}_{2}}$ into oxygen atoms, which react with ${{O}_{2}}$ to form ${{O}_{3}}$.
\[\begin{align}
& {{O}_{2}}\xrightarrow{hv}O+O \\
& O+{{O}_{2}}\to {{O}_{3}} \\
\end{align}\]
Note: Note that ozone is a homonuclear molecule as it contains only one type of atoms. It is easier to count the total number of atoms in homonuclear molecules than in heteronuclear molecules, which contain more than one type of atoms. So in such cases, divide the mass of the molecule by the mass of the atom, of which you want to find the atomicity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




