
Atomicity of ammonium phosphate molecule is:
Answer
606.9k+ views
Hint – We will start this question by writing down some definitions, like, molecules and its categories, atomicity and we will also make structure of ammonium phosphate to make everything clear. Then we will find the atomicity of ammonium phosphate to get the required answer.
Complete step-by-step answer:
We know that,
A molecule is the smallest particle of an element or a compound which can exist freely in nature.
Molecules can be classified into two categories:
(i) Molecules of elements
(ii) Molecules of compounds
(i) Molecules of elements – They are made up of only one kind of atoms, they are called homoatomic or homonuclear molecules. Further depending upon whether the molecule contains only one, two, three, etc., atoms are called monatomic, diatomic, triatomic, etc.
(ii) Molecules of compounds – They are made up of atoms of different elements and hence are called heteroatomic or heteronuclear molecules. They may be diatomic, triatomic, etc., depending upon the number of atoms present in one molecule of the compounds.
Also,
Atomicity can be defined as the total number of atoms of which a molecule is composed.
We know that ammonium phosphate is a heteronuclear molecule and for heteronuclear molecules atomicity simply means the total number of atoms present in the given molecule.
Now, we have to find out the atomicity of ammonium phosphate for which we must know the molecular formula ammonium phosphate and its structure.
The molecular formula of ammonium phosphate is ${\left( {N{H_4}} \right)_3}P{O_4}$ and its structure is as follows:
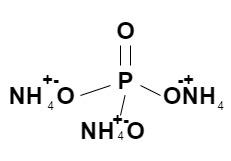
We can see in the molecular formula and in the structure that there are four different elements in ammonium phosphate, i.e., Nitrogen, Hydrogen, Phosphors and Oxygen.
Number of Nitrogen atoms in ammonium phosphate = 3
Number of Hydrogen atoms in ammonium phosphate = 12
Number of Phosphors atoms in ammonium phosphate = 1
Number of Oxygen atoms in ammonium phosphate = 4
Therefore, atomicity of ammonium phosphate $\left( {{{\left( {N{H_4}} \right)}_3}P{O_4}} \right)$= 3 + 12 + 1 + 4
= 20
Hence, the atomicity of ammonium phosphate molecules is 20.
Note – Ammonium phosphate is manufactured by mixing together ammonium phosphate sulphate and urea in a molten condition. It is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant.
Complete step-by-step answer:
We know that,
A molecule is the smallest particle of an element or a compound which can exist freely in nature.
Molecules can be classified into two categories:
(i) Molecules of elements
(ii) Molecules of compounds
(i) Molecules of elements – They are made up of only one kind of atoms, they are called homoatomic or homonuclear molecules. Further depending upon whether the molecule contains only one, two, three, etc., atoms are called monatomic, diatomic, triatomic, etc.
(ii) Molecules of compounds – They are made up of atoms of different elements and hence are called heteroatomic or heteronuclear molecules. They may be diatomic, triatomic, etc., depending upon the number of atoms present in one molecule of the compounds.
Also,
Atomicity can be defined as the total number of atoms of which a molecule is composed.
We know that ammonium phosphate is a heteronuclear molecule and for heteronuclear molecules atomicity simply means the total number of atoms present in the given molecule.
Now, we have to find out the atomicity of ammonium phosphate for which we must know the molecular formula ammonium phosphate and its structure.
The molecular formula of ammonium phosphate is ${\left( {N{H_4}} \right)_3}P{O_4}$ and its structure is as follows:

We can see in the molecular formula and in the structure that there are four different elements in ammonium phosphate, i.e., Nitrogen, Hydrogen, Phosphors and Oxygen.
Number of Nitrogen atoms in ammonium phosphate = 3
Number of Hydrogen atoms in ammonium phosphate = 12
Number of Phosphors atoms in ammonium phosphate = 1
Number of Oxygen atoms in ammonium phosphate = 4
Therefore, atomicity of ammonium phosphate $\left( {{{\left( {N{H_4}} \right)}_3}P{O_4}} \right)$= 3 + 12 + 1 + 4
= 20
Hence, the atomicity of ammonium phosphate molecules is 20.
Note – Ammonium phosphate is manufactured by mixing together ammonium phosphate sulphate and urea in a molten condition. It is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




