
Assign ON to atoms of only those elements which undergo ON change in the following redox reactions and then balance the equation.
${{H}_{2}}S+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\to KHS{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+S+{{H}_{2}}O$
Answer
512.7k+ views
Hint: First, balance all the elements except hydrogen and oxygen atoms, so in this reaction balance sulfur, chromium, potassium before balancing hydrogen and oxygen. For finding the oxidation state of the elements, take the oxidation state of hydrogen and potassium as +1 and oxidation number oxygen as -2.
Complete answer:
The given reaction is:
${{H}_{2}}S+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\to KHS{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+S+{{H}_{2}}O$
The oxidation number of H in ${{H}_{2}}S$ is +1 and the oxidation number of S is -2.
The oxidation number of K in ${{K}_{2}}C{{r}_{2}}{{O}_{7}}$ is +1, the oxidation number of Cr is +6 and the oxidation number of O is -2.
The oxidation number of H in ${{H}_{2}}S{{O}_{4}}$ is +1 and the oxidation state of $SO_{4}^{2-}$ is -2.
The oxidation number of K and H in $KHS{{O}_{4}}$ is +1, and the oxidation state of $SO_{4}^{2-}$ is -2.
The oxidation number of Cr in $C{{r}_{2}}{{(S{{O}_{4}})}_{3}}$ is +3, and the oxidation state of $SO_{4}^{2-}$ is -2.
Since the S is in the elemental form its oxidation state will be 0.
The oxidation state of H in ${{H}_{2}}O$ is +1 and the oxidation state of O is -2.
So, in the reaction, the oxidation state of chromium decreases from +6 to +3, and the oxidation state of sulfur increases from -2 to 0. Therefore, ${{H}_{2}}S$ is the reducing agent and ${{K}_{2}}C{{r}_{2}}{{O}_{7}}$ is the oxidizing agent.
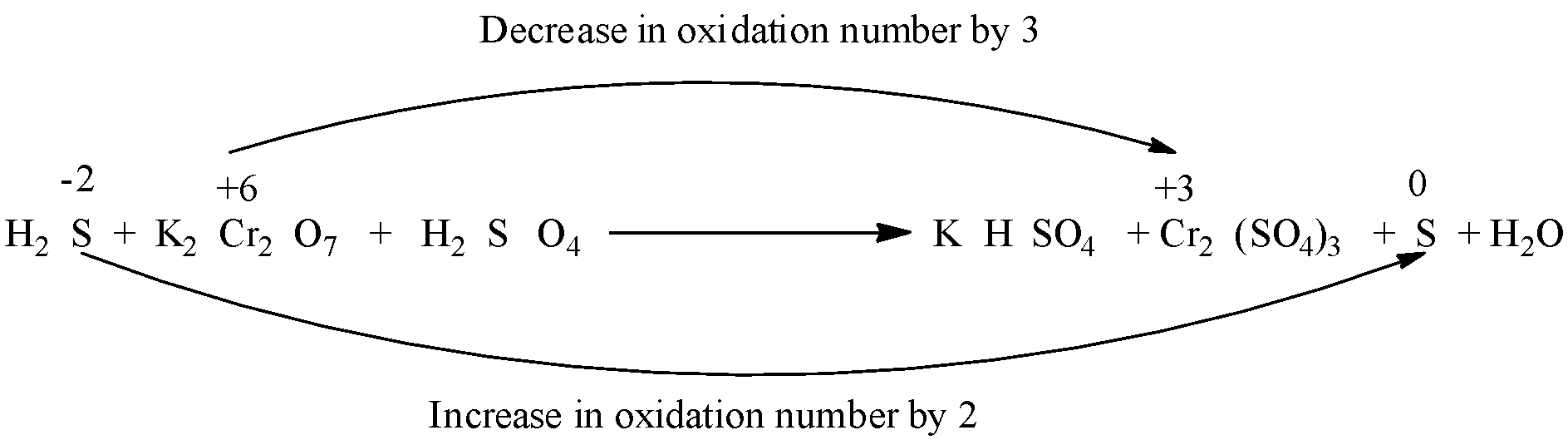
First, balance all the elements except hydrogen and oxygen atoms, so in this reaction balance sulfur, chromium, potassium before balancing hydrogen and oxygen.
So, the balanced equation will be:
$3{{H}_{2}}S+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+5{{H}_{2}}S{{O}_{4}}\to 2KHS{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+3S+7{{H}_{2}}O$
Note:
If the equation is in an acidic medium then to balance the hydrogen atoms, we can add hydrogen ions (${{H}^{+}}$) and if the equation is in basic medium then to balance hydrogen and oxygen we can add hydroxyl ions ($O{{H}^{-}}$).
Complete answer:
The given reaction is:
${{H}_{2}}S+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+{{H}_{2}}S{{O}_{4}}\to KHS{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+S+{{H}_{2}}O$
The oxidation number of H in ${{H}_{2}}S$ is +1 and the oxidation number of S is -2.
The oxidation number of K in ${{K}_{2}}C{{r}_{2}}{{O}_{7}}$ is +1, the oxidation number of Cr is +6 and the oxidation number of O is -2.
The oxidation number of H in ${{H}_{2}}S{{O}_{4}}$ is +1 and the oxidation state of $SO_{4}^{2-}$ is -2.
The oxidation number of K and H in $KHS{{O}_{4}}$ is +1, and the oxidation state of $SO_{4}^{2-}$ is -2.
The oxidation number of Cr in $C{{r}_{2}}{{(S{{O}_{4}})}_{3}}$ is +3, and the oxidation state of $SO_{4}^{2-}$ is -2.
Since the S is in the elemental form its oxidation state will be 0.
The oxidation state of H in ${{H}_{2}}O$ is +1 and the oxidation state of O is -2.
So, in the reaction, the oxidation state of chromium decreases from +6 to +3, and the oxidation state of sulfur increases from -2 to 0. Therefore, ${{H}_{2}}S$ is the reducing agent and ${{K}_{2}}C{{r}_{2}}{{O}_{7}}$ is the oxidizing agent.

First, balance all the elements except hydrogen and oxygen atoms, so in this reaction balance sulfur, chromium, potassium before balancing hydrogen and oxygen.
So, the balanced equation will be:
$3{{H}_{2}}S+{{K}_{2}}C{{r}_{2}}{{O}_{7}}+5{{H}_{2}}S{{O}_{4}}\to 2KHS{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+3S+7{{H}_{2}}O$
Note:
If the equation is in an acidic medium then to balance the hydrogen atoms, we can add hydrogen ions (${{H}^{+}}$) and if the equation is in basic medium then to balance hydrogen and oxygen we can add hydroxyl ions ($O{{H}^{-}}$).
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




